| Citation: | Roselvy Juárez, Eduardo Chacón-Madrigal, Luis Sandoval. 2020: Urbanization has opposite effects on the territory size of two passerine birds. Avian Research, 11(1): 11. DOI: 10.1186/s40657-020-00198-6 |
Urban expansion has been identified as one of the leading drivers of biodiversity change or loss. For birds, urbanization is specifically related to survival, breeding success, and territory size. Understanding how different birds adjust territory size in response to urbanization is essential for their conservation in urban environments and to better understand why some species are lost and others persist under this condition. We evaluated the effect of urbanization on the territory size of an urban avoider species, White-eared Ground-Sparrow (Melozone leucotis), and an urban adapter species, House Wren (Troglodytes aedon), at five Costa Rican sites.
We measured the size of 30 ground-sparrow and 28 wren territories using a total of 296 h of observation. We followed each individual for at least 1 h per day for at least 2 days of two consecutive years, and geo-referenced their locations. Territory size was estimated using the minimum convex polygon method. We measured the urban surfaces (roads, buildings, any other paved area, soccer fields, lawns, and gardens with short grass) within territories.
Ground-sparrow territories were larger at the highly urbanized site than at the non-urbanized site. Wren territories were larger at the low urbanized site than at the highly urbanized site. We found a positive relationship between urban surface and territory size for the ground-sparrow, but not for the wren.
Our results showed that not all birds adjust territory size in the same way in response to urbanization. We showed that urban avoiders probably need to defend larger territories in urban environments to find all the resources required to survive because urban environments may provide insufficient resources such as food or shelter. Urban adapters on the other hand defend smaller territories in urban environments because even small territories may provide sufficient resources. These results suggest specific behavioral adaptations developed by Neotropical birds inhabiting urban environments.
The territory is defined as an area protected by one or several individuals of the same species against other individuals of the same species (although some species defend territories against several species) over a time period (Tinbergen 1936; Brown 1969; Salomonson and Balda 1977). Birds may establish territories during the breeding season (Brown 1963), or year-round (Woltmann and Sherry 2011; Duca and Marini 2014; Holland et al. 2017). During the breeding season, territories may provide places for mate attraction, food, and nesting (Nice 1941; Osborne and Bourne 1977; Woltmann and Sherry 2011). Outside the breeding season, territories may provide food and roosting places (Nice 1941; Salomonson and Balda 1977; Kraaijeveld and Dickinson 2001).
Birds territories are defended by males or females alone (Osborne and Bourne 1977; Møller 1990; Weaving et al. 2014), pairs (Tinbergen 1936; Brown 1963), or social groups (Brown 1969; Kraaijeveld and Dickinson 2001; Duca and Marini 2014). For example, males of Northern Cardinal (Cardinalis cardinalis) defend territories against other males during the breeding season to secure access to females, but they do not do so outside the breeding season (Halkin and Linville 1999). In Chestnut-backed Antbird (Myrmeciza exsul), both males and females defend territories year-round to secure food resources and partners (Halkin and Linville 1999). In Western Bluebird (Sialia mexicana), outside the breeding season, groups of up to 12 individuals defend territories against other individuals to protect food resources (Kraaijeveld and Dickinson 2001).
Territory size and shape are not static over time and may change according to resource abundance and the energy invested in defense (Hixon et al. 1983; Møller 1990; Butchart et al. 1999). Therefore, a balance is expected between territory size and resource abundance or territory size and the time that each individual invests in territorial defense, given that territory size is adjusted according to the benefits obtained from the defense (Brown 1969; Salomonson and Balda 1977; Hixon et al. 1983). For example, in Rufous Hummingbird (Selasphorus rufus) there is a positive correlation between territory size and weight increase, until a threshold over which the increase stops (Carpenter et al. 1983).
In urban environments, some resources may be more abundant than others but of inferior quality (Mennechez and Clergeau 2006). For example, human waste is more abundant in cities, benefiting species that use this resource as part of the diet, resulting in smaller territories inside cities compared to rural or natural habitats (McGowan 2001; Lowry et al. 2013). The opposite is also true, because in urban environments, reduced abundance of natural or specialized resources as specific fruits, flowers, or insects, encourages birds feeding on those resources to defend larger territories to meet their requirements (Mennechez and Clergeau 2006; Weaving et al. 2014). Even generalist species that feed on human waste or human-supplied food may depend on high-quality natural resources to supply all their nutritional and energetic requirements, prompting their necessity to defend larger territories to meet them (Mennechez and Clergeau 2006; Lowry et al. 2013). In general, it is expected that urban avoider species (i.e. species sensitive to human-produced changes in the landscape and with specialized diets) have larger territories inside cities than urban adapter species (i.e. species able to use additional resources provided by humans) (Salomonson and Balda 1977; Marzluff et al. 2016; Ruiz-Sánchez et al. 2017). Considering that not all species respond equally to urbanization and that territory size is not static over time (Møller 1990; MacDougall-Shackleton and Robertson 1995; Holland et al. 2017), we need to understand how different species adjust the size of their territories in response to changes in resource availability generated by humans, to maintain diversity in urban environments.
Our main objective in this study is to test the structural cues hypothesis (Smith and Shugart 1987), which proposes that bird territory size is related to structural habitat features (e.g. urban and natural habitat) as an indicator of resource supply (i.e. food and nesting sites). More specifically, we evaluated two objectives. First, we evaluated if the degree of urbanization is associated with territory size in White-eared Ground-Sparrow (Melozone leucotis) and House Wren (Troglodytes aedon). Second, we evaluated if House Wren territory size varies throughout the year along an urban–rural gradient. We selected White-eared Ground-Sparrow and House Wren as model species because both occur sympatrically along urban–rural gradients in the Central Valley of Costa Rica and both show different affinities for urban environments. White-eared Ground-Sparrow inhabits dense thickets of humid and young secondary forest, coffee plantations, and densely vegetated gardens (Stiles and Skutch 1989; Sandoval and Mennill 2012; Sandoval et al. 2015). Both pair members, male and female, defend the same territory year-round during multiple years (Sandoval et al. 2015), but territory defense appears to be more pronounced during the breeding season when males sing solo songs (Sandoval et al. 2016). Conversely, House Wren is found in open or semi-open areas, from forest edges to urban areas with scattered vegetation (Skutch 1953; Howell and Webb 1995; Johnson 2014). Both male and female House Wren stay in the territory year-round and males sing all year to defend their first territory for life (Skutch 1953; Johnson 2014). Although both species occur throughout the Central Valley of Costa Rica, they are not equally abundant along its urban–rural gradient. White-eared Ground-Sparrow is an urban avoider and achieves moderate densities in natural habitats (Blair 1996; Marzluff and Rodewald 2008). House Wren is an urban adapter and can be common in urban environments with forest patches (Blair 1996; Marzluff and Rodewald 2008).
White-eared Ground-Sparrow and House Wren differ in the benefits obtained by using infrastructure or landscape produced by urbanization. For example, House Wrens forage in the majority of substrates available if these contain invertebrates, and build nests in buildings and other cavities provided by humans (Skutch 1953; Johnson 2014), so they may only need to defend a small space in order to find food and other resources. White-eared Ground-Sparrows, on the other hand, feed mostly in humid leaf litter and require dense thickets to build nests (Sandoval and Mennill 2012), so they may need to defend more space to find food and other resources. Thus, for White-eared Ground-Sparrow, we expected to find larger territories at the urban sites than at the rural ones; meanwhile, for House Wren, we expected to find the opposite pattern. Territory size changes over time according to the status of the breeding cycle; to secure breeding success males need to defend larger territories to protect food resources and mates (Møller 1990; MacDougall-Shackleton and Robertson 1995; Holland et al. 2017). Thus, for House Wren, we expected to find smaller territories during the nonbreeding season than during the breeding season.
We conducted this study at five sites within the Central Valley of Costa Rica. (1) Universidad de Costa Rica (9.9378° N, 84.0498° W, Coordinate Reference System: WGS84). The habitat at this location is 1.5 ha forest reserve surrounded by gardens, isolated trees, and buildings. The reserve is a 50-years old secondary growth forest composed of trees such as Ficus spp. (Moraceae), Erythrina poeppigiana (Fabaceae), and Cordia eriostigma (Boraginaceae) with an understory of palms, dense bushes, and some vines (Nishida et al. 2009). Birds at this site are exposed to high human disturbance. (2) Ciudad de la Investigación (9.9390° N, 84.0438° W). The habitat at this location is a mix of a riparian strip, gardens, isolated trees, and buildings. The riparian strip is dominated by trees such as Ficus spp. (Moraceae) and Erythrina poeppigiana (Fabaceae). Birds at this site are exposed to moderate to high human disturbance. (3) Instalaciones Deportivas (9.9452° N, 84.0449° W). The habitat at this location is a mix of small patches of secondary forest mixed with dense thickets of native herbs and exotic grasses, gardens with isolated trees, and some buildings (Biamonte et al. 2011). The secondary forest is dominated by trees such as Ficus spp. (Moraceae), Inga spp., and Cedrela odorata, with an understory of dense bushes and vines. Birds at this site are exposed to moderate human disturbance. (4) Jardín Botánico Lankester (9.8391° N, 83.8903° W). The habitat at this location is a mix of a medium-sized secondary forest, with gardens and a few buildings. The forest is dominated by Ficus spp. (Moraceae) trees with an understory of dense bushes and vines. Birds at this site are exposed to moderate to low human disturbance. (5) Getsemaní de Heredia (10.0329° N, 84.1131° W). The habitat at this location is a mix of a large secondary forest and an abandoned shaded coffee plantation. The forest is dominated by trees such as Ficus spp. (Moraceae), Inga spp. (Fabaceae), Lonchocarpus spp. (Fabaceae), Erythrina spp. (Fabaceae), and Musa spp. plants with an understory of dense bushes and vines. Birds at this site are exposed to very low human disturbance. Hereafter we will refer to these sites as highly urbanized, medium–high urbanized, medium–low urbanized, low urbanized, and non-urbanized sites respectively. These sites were selected because they are exposed to a gradient of anthropogenic disturbance (e.g. human presence, light pollution, and noise) from the highly urbanized to the non-urbanized site, which positively correlates with urban surface (Blair 1996; Warren et al. 2006). We did not sample White-eared Ground-Sparrow at the medium–high urbanized site because only one pair was present in a site of difficult access; nor House Wren at the non-urbanized site because their density was very low and the individuals were hard to follow and observe inside their habitats. For this reason, we excluded these sites from the study for each species.
At each site, we captured and color-banded at least five males (House Wren) or pairs (White-eared Ground-Sparrow). We followed them during two consecutive breeding seasons. For House Wrens, we only marked males because only they defend territories (Johnson 2014). In total, we followed 32 pairs of White-eared Ground-Sparrows (highly urbanized site = 5, medium–low urbanized site = 6, low urbanized site = 13, and non-urbanized site = 8; 17 pairs were followed both years) and 30 House Wrens (highly urbanized site = 7, medium–high urbanized site = 7, medium–low urbanized site = 6, and low urbanized site = 10; 21 individuals were followed both years). At the beginning of each breeding season (April to July 2015 and 2016), we followed focal individuals on two different days for 1 h per day, between 0500 and 0700 h, when both species were typically more active and easier to observe, for a combined total of 200 h of observation. We also monitored 24 House Wren territories outside the breeding season (from mid-October to the end of November, in 2015 and 2016), on two different days for 1 h per day, between 0500–0700 h, for a combined total of 96 h of observation. While White-eared Ground-Sparrows maintain territories year-round, we studied them only during the breeding season, when their territorial behavior is more pronounced (Sandoval et al. 2015; 2016). Outside the breeding season, pairs are shy and reduce the number of interactions with neighbors, and males do not sing from territory edge perches (Sandoval et al. 2016), rendering the collection of enough data points to estimate territory size difficult. We avoided disturbing focal individuals by following them from a moderate distance (average of 12 m, range: 8–16 m), and by wearing unobtrusive clothing. At the end of every hour of observation, we collected the coordinates of each site used by each focal individual for singing using a Global Positioning System device (GPS, GARMIN model map 62, accuracy = 3 m). Both species sing to defend the territory and the ground-sparrow also uses duets for a similar purpose (Skutch 1953; Johnson 2014; Sandoval et al. 2016). We collected the coordinates only when the GPS precision indicated ≤ 4 m. For White-eared Ground-Sparrow, the number of coordinates collected per territory ranged from 5 to 25 (mean ± SE: 9 ± 0.6); for House Wren, the number of coordinates collected per territory ranged from 5 to 24 (9 ± 0.4).
Because the position of any locality along a landscape gradient not necessarily indicates its degree of urbanization, we corroborated our estimations measuring the proportion of urban surfaces (i.e. roads, buildings, any other paved area, soccer fields, lawns, and gardens with short grass) within a 500 m radius circle around each site sampled. We used as circle center the centroid of all coordinates collected for each territory. Within each circle, we measured the urban surface area by manually drawing polygons over satellite images using the land area calculator in Google Earth Pro. The spatial resolution of the satellite images used was 46 cm, with an eye altitude of 1.30 km. For this analysis, we followed the methods used by Greggor et al. (2016).
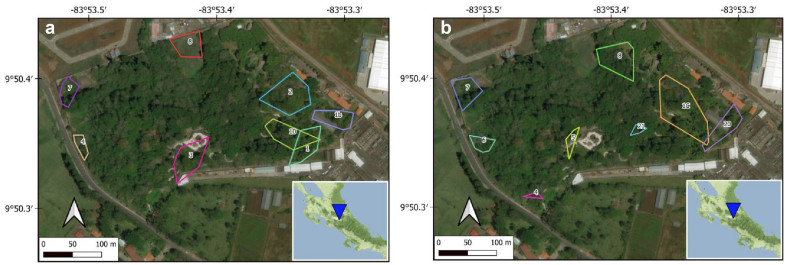
We estimated the territory size of each focal individual (wren) or focal pair (ground-sparrow); using the minimum convex polygon of the GPS coordinates collected during each season per year. This technique of territory estimation has been used to estimate territory sizes in other bird species such as Wilson's Warbler (Cardellina pusilla), Chestnut-crowned Antpitta (Grallaria ruficapilla), and Red-eyed Vireo (Vireo olivaceus; Kattan and Beltran 2002; Marshall and Cooper 2004; Ruiz-Sánchez et al. 2017). We used the "adehabitat" package in R (Calenge 2006), to estimate territory size as the minimum convex polygon using the sets of coordinates of each focal pair. We calculated the minimum convex polygon only for individuals for which at least five sets of coordinates per season (wren) or year (ground-sparrow) were collected (Fig. 1a, b). The average number of coordinates used to estimate the minimum convex polygon is within the minimum required to implement this method to estimate territory size, as well as within the number used in other studies (Marshall and Cooper 2004; Ruiz-Sánchez et al. 2017).
We classified the habitats available in each territory using two categories: (1) urban surface (impervious surface: roads, buildings, and any other paved area; and landscaped surface: lawns, soccer fields, and open gardens with short grass) and (2) natural surface (thickets and secondary forest with well-developed understory). We measured the urban surface areas inside every territory by manually drawing polygons of each type of surface area using satellite images and the land area calculator in Google Earth Pro. Additionally, where necessary, we used field information to distinguish between natural and landscaped surface to draw the polygons. We used satellite images from April 2015 and May 2016, with a spatial resolution of 46 cm, with an eye altitude of 1.30 km.
We conducted a linear mixed-effects model to test if the territory size of White-eared Ground-Sparrow varies between the four studied sites (i.e. highly urbanized, medium–low urbanized, low urbanized, and non-urbanized sites). In this analysis, we used the site as the fixed factor and territory size per year per pair as the response variable. We used the pair identity as a random factor to account for the fact that each pair was sampled repeatedly. After the linear mixed-effects model proved that site was a significant factor, we followed up with Tukey's HSD post hoc tests to determine where the differences were. We conducted a linear analysis to test the structural cues hypothesis. We specifically tested if territory size increased with the proportion of urban surface, rather than total urban surface. This analysis corrects for differences in territory size between pairs, allowing us to evaluate if this variable influences territory size. We used parametric tests because we sampled more than 20 territories for this species, and after conducting a square root transformation on territory size, our data follow a normal distribution (Shapiro-Wilks test: W = 0.95, P = 0.12).
We conducted another linear mixed-effects model to test if House Wren territory size varies between season and among the four studied sites (i.e. highly urbanized, medium–high urbanized, medium–low urbanized, and low urbanized sites). In this analysis, we included two fixed factors without interaction: season and site. The response variable was the territory size per season per individual. We used the individual identity as a random factor to account for the fact that each male was sampled repeatedly. Likewise, after the linear mixed-effects model showed that site was a significant factor, we used Tukey's HSD post hoc tests to determine differences between sites. Similar to the model for the White-eared Ground-Sparrow, we then conducted a linear regression to test the structural cues hypothesis. We used parametric tests because tests showed normality for House Wren data (Shapiro-Wilks test: W = 0.96, P = 0.40). In both linear analyses, we used as the Y variable the average of the territory size per pair (White-eared Ground-Sparrow) or per individual (House Wren). We used the R language and environment 3.3.3 for all statistical analyses (R Core Team 2017). We report average ± SE for all our measurements.
Based on our 500 m radius circle, our urbanization analysis supports the classification of the study sites from more to less urban sites: urban surface comprised 66% of the highly urbanized site, 57% of the medium–high urbanized site, 52% of the medium–low urbanized site, 22% of the low urbanized site, and only 1% of the non-urbanized site. We estimated territory size for 30 White-eared Ground-Sparrow territories and 28 House Wren territories for both years combined. These data differ from the number of territories monitored (32 and 30 respectively) because for some focal individuals we were unable to collect a minimum of five sets of coordinates per season (wren) or year (ground-sparrow), preventing us to estimate territory size. White-eared Ground-Sparrow territory size was 0.18 ± 0.03 ha, n = 30. House Wren territory size was 1.8 times larger during the breeding season (0.11 ± 0.01 ha, n = 27) than during the non-breeding (0.06 ± 0.01 ha, n = 24; F1, 55 = 17.24, P < 0.001).
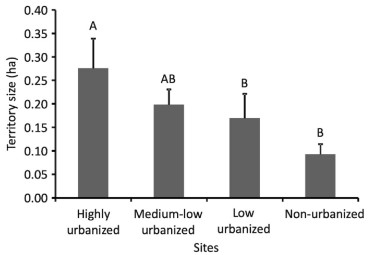
White-eared Ground-Sparrow territory size varied according to the degree of urbanization (F3, 27 = 2.38, P = 0.02, Fig. 2). Territories were 3.1 times larger at the highly urbanized site (0.28 ± 0.04 ha, n = 5) in comparison to the non-urbanized site (0.09 ± 0.02 ha, n = 6; post hoc test: P = 0.01, Fig. 2). We also found this pattern between the highly urbanized site and the low urbanized site, where territories were 1.8 times smaller (0.09 ± 0.01 ha, n = 6; post hoc test: P = 0.03, Fig. 2). For all other pair comparisons between territory sizes according to the degree of urbanization per studied site, we did not find any difference (post hoc test: P > 0.06 for all comparisons, Fig. 2). White-eared Ground-Sparrow territory size increased as the proportion of urban surface inside the territory increased (r2 = 0.15, P = 0.03, Fig. 3).
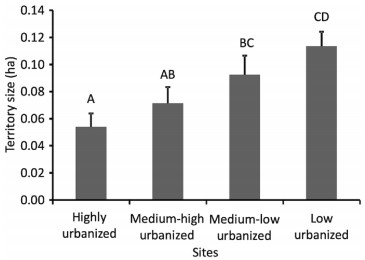
House Wren territory size varied according to the degree of urbanization (F3, 24 = 5.75, P = 0.004, Fig. 4). We found 2.2 times smaller territories at the highly urbanized site in comparison to the low urbanized site (0.05 ± 0.01 ha, n = 7; 0.12 ± 0.01 ha, n = 9; post hoc test: P = 0.001, Fig. 4). We also found this pattern between the highly urbanized site and the medium–low urbanized site, where territories were 1.8 times smaller (0.09 ± 0.01 ha, n = 6; post hoc test: P = 0.03, Fig. 4) and between the medium–high urbanized site and the low urbanized site, where territories were 1.6 times smaller (0.07 ± 0.01 ha, n = 6; post hoc test: P = 0.008, Fig. 4). For all other pair comparisons between territory sizes according to the degree of urbanization per studied site, we did not find any difference (post hoc test: P > 0.06 for all comparisons, Fig. 4). House Wren territory size was not related to the proportion of urban surface inside the territory (r2 = 0.00, P = 0.91, Fig. 5).
We found clear differences in the variation of territory size according to the degree of urbanization in the two studied species. White-eared Ground-Sparrow had larger territories and House Wren had smaller territories at the highly urbanized site. Feeding habits may be the factor driving this pattern. For certain species like some raptors and waterbirds, urban environments provide more food (Frixione et al. 2012; Boggie and Mannan 2014), while for others that require some specialized food at least in some part of their life, like European Starling (Sturnus vulgaris), urban environments may not provide all necessary dietary components (McGowan 2001; Mennechez and Clergeau 2006). Flexibility in feeding habits may allow urban adapters such as House Wren to have smaller territories in urban environments because they can use multiple food and habitat resources (Skutch 1953; Lowry et al. 2013). Meanwhile, a species with more limited feeding habits (e.g. an obligate ground feeder such as White-eared Ground-Sparrow) may need larger territories to obtain all the required food. Although territory size is related to body size, with larger birds defending larger territories (Price 1984; Weaving et al. 2014), a pattern also confirmed in this study, this cannot explain the opposite patterns that we found for our two focal species along an urban gradient.
Additionally, resource availability (e.g. nesting places) may be another factor driving the opposite pattern of the effects of urbanization on the territory size of ground-sparrows and wrens. For secondary cavity-nesting species like House Wren (Skutch 1953; Johnson 2014), urban environments may increase the availability of places for nesting since buildings and other human structures provide many holes to nest (Skutch 1953; Emlen 1974). Conversely, for species that nest on the ground and in dense thickets like White-eared Ground-Sparrow (Sandoval and Mennill 2012), urban environments may offer fewer nesting places (Emlen 1974; Mills et al. 1989; Vignoli et al. 2013). The positive relationship we found between ground-sparrow territory size and the proportion of urban surface provides additional support for the relevance of resource availability in determining territory size (Eberhard and Ewald 1994; Marshall and Cooper 2004; Weaving et al. 2014; Ruiz-Sánchez et al. 2017), because individuals inhabiting highly urbanized territories may need to protect larger areas in order to secure all necessary resources to survive and reproduce.
Density is another factor that may determinate territory size for both species (Brown 1969; Boggie and Mannan 2014; Weaving et al. 2014). It is expected that an urban avoider like White-eared Ground-Sparrow achieves its highest abundances in more natural environments (Blair 1996; Marzluff and Rodewald 2008). Therefore, at urban sites, where ground-sparrows apparently are less abundant (Biamonte et al. 2011), they have more area available, allowing them to defend larger territories. An urban adapter like House Wren, on the other hand, achieves its highest abundance in suburban and urban environments with some forest patches (Skutch 1953; Blair 1996; Boggie and Mannan 2014), where they defend smaller territories compared to natural sites (McGowan 2001; Johnson 2014). The fact that in urban environments ground-sparrows had the largest territories while wrens had the smallest territories compared to the low-urbanized environments provides support for the influence of population abundance in the determination of territory size in these two passerines. However, it is also plausible that the difference in territory size between urban and low-urbanized environments is driving the difference of densities between those environments, as has been suggested to explain differences in densities of several species of antpittas (Kattan and Beltran 2002).
House Wren territories in the Costa Rican Central Valley were larger during the breeding season than outside the breeding season (approximately 83% larger). In line with our results, larger territories during the breeding season have been reported in several passerine species like Yellowhammer (Emberiza citronella), Barn Swallow (Hirundo rustica), and Wilson's Warbler (e.g. Møller 1990; Ruiz-Sánchez et al. 2017) and non-passerine species like Little Owl (Athene noctua) and Common Cuckoo (Cuculus canorus; e.g. Finck 1990; Williams et al. 2016). For wrens, similar results have been reported in Pacific Wren (Troglodytes pacificus; Hejl et al. 2002) and Marsh Wren (Cistothorus palustris; Kroodsma and Verner 2013). Larger territories during the breeding season, especially at the beginning when females are more fertile, may help males to reduce the risk of the female being fertilized by another male, and therefore lose reproduction opportunities (Møller 1990, 1992). In polygynous species like House Wren, large territories during the breeding season also may allow males more mating opportunities because this may increase the probability of more females settling to breed (Dunn 1992; Johnson 2014; Alcock 2016). Additionally, larger territories may be necessary to access the food resources that a breeding pair requires (Salomonson and Balda 1977; Ruiz-Sánchez et al. 2017). This, in turn, reduces the time that nests are left unattended, thus decreasing nest predation (Beissinger et al. 1998), or infanticide, as has been reported in unattended House Wren nests (Freed 1986; Kermott et al. 1991; Kattan 2016). Larger territories also reduce the need to enter another pair's territory to obtain food, and this reduces territorial conflicts that may result in physical damage to the parents.
Our results regarding the effects of urbanization on the territory size of White-eared Ground-Sparrow and House Wren showed that urbanization has opposite effects on the territory size of these two passerines with different responses to urban development: White-eared Ground-Sparrow has larger territories in urban environments while House Wren shows the opposite pattern. Our results of the annual variation in House Wren territory size support the defense of larger territories during the breeding season, and this may help House Wren males to secure the highest reproductive success. Larger territories may ensure mate fidelity and also prevent the premature death of the pair's offspring by increasing nest and juvenile protection. These results are important for urban management practices because they provide evidence about the habitat structure (proportion of urban and natural surface) and size necessary for the conservation of species with different degree of tolerance to urban development. Our results also highlight the relevance of dense thicket, a habitat generally underappreciated, but important for the conservation of urban adapter and avoider species alike (Sandoval et al. 2019). For an urban avoider species such as White-eared Ground-Sparrow whose natural habitat includes dense understories (Sandoval and Mennill 2012; Sandoval et al. 2016), dense thickets inside urban areas constitute an irreplaceable trophic substratum for roosting, breeding and feeding. This habitat also provides resources for other species of birds, mammals, and arthropods specialized or restricted to this habitat inside urban areas to survive (Sandoval et al. 2019).
We are grateful to C. Méndez, M. Villarreal, and O. Sandoval for field assistance. We thank M. Fernández, Eben Goodale, and two anonymous reviewers for helpful comments on early drafts of the manuscript. We thank J. van Dort for useful comments on a previous version of the manuscript and for improving English writing and grammar.
LS secured funding for field work. RJ and LS conceived the idea and conzducted the research. RJ, LS, and ECM analyzed the data, and wrote and edited the paper. All authors read and approved the final manuscript.
RJ gratefully acknowledges a grant in the form of the scholarship Julio E. Sánchez para el Avance de la Ornitología from Unión de Ornitólogos de Costa Rica. LS would like to thank Escuela de Biología, Universidad de Costa Rica, for the partial time support, and to Vicerrectoría de Investigación for the support under the project number B5241 Ecología de la comunicación acústica en aves de ambientes urbanos. None of our funders had any input into the content of the manuscript, nor did they require the approval of the manuscript before submission.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
This study was approved by Vicerrectoria de Investigación, Universidad de Costa Rica number N-111-B5-241. Birds were handled only by two of the authors, both of whom have demonstrable experience banding birds.
Not applicable.
The authors declare that they have no competing interests.
|
Alcock J. Avian mating and social behavior. In: Lovette IJ, Fitzpatrick JW, editors. The Cornell Lab of Ornithology Handbook of bird biology. Hoboken: Wiley Press; 2016. p. 313–53.
|
|
Brown JL. Territorial behavior and population regulation in birds: a review and re-evaluation. Wilson Bull. 1969;81:293–329.
|
|
Freed LA. Territory takeover and sexually selected infanticide in tropical house wrens. Behav Ecol Sociobiol. 1986;19:197–206.
|
|
Howell SNG, Webb S. A guide to the birds of Mexico and northern Central America. New York: Oxford University Press; 1995.
|
|
Marzluff JM, Rodewald A. Conserving biodiversity in urbanizing areas: nontraditional views from a bird's perspective. Cit Environ. 2008;1:1–27.
|
|
McGowan KJ. Demographic and behavioral comparisons of suburban and rural American Crows. In: Marzluff JM, Bowman R, Donelly R, editors. Avian ecology and conservation in an urbanizing world. Norwell: Kluwer Academic Press; 2001. p. 365–81.
|
|
Sandoval L, Mennill DJ. Breeding biology of White-eared Ground-sparrow (Melozone leucotis), with a description of a new nest type. Ornitol Neotrop. 2012;23:225–34.
|
|
Stiles FG, Skutch AF. A guide to the birds of Costa Rica. Ithaca: Cornell University Press; 1989.
|
| 1. | Berlusconi, A., Castiglione, G., Clerici, E. et al. Heterospecific territorial defense in tit species varies according to breeding habitat overlap. Behavioral Ecology, 2025, 36(4): araf082. DOI:10.1093/beheco/araf082 |
| 2. | Cueva, L., Fuchs, E.J., Barrantes, G. et al. Effect of Spatial and Temporal Urban Isolation on the Genetic Diversity, Acoustic Variation, and Morphological Characteristics of an Urban Survivor Bird Species. Ecology and Evolution, 2025, 15(4): e70972. DOI:10.1002/ece3.70972 |
| 3. | Menon, M.. Urban Birds and Adaptive Behaviours. Animal Behavior in the Tropics Vertebrates, 2025. DOI:10.1007/978-981-97-7324-4_17 |
| 4. | Dayananda, S.K., Athukorala, H.F., Peabotuwage, I. et al. Rubber plantations are impermeable to an avian understory specialist in Sri Lanka. Movement Ecology, 2024, 12(1): 47. DOI:10.1186/s40462-024-00484-8 |
| 5. | Sheng, Y., Lu, M., Bai, J. et al. Ecological drivers of nesting behavior in a subtropical city: An observational study on spotted doves. Ecology and Evolution, 2024, 14(7): e11655. DOI:10.1002/ece3.11655 |
| 6. | Villarreal, M., Barrantes, G., Sandoval, L. SONG FREQUENCY SHIFTS IN AN URBAN BIRD SPECIES OPTIMIZE ACOUSTIC TRANSMISSION INSIDE NOISY URBAN AREAS | [Los cambios en la frecuencia del canto en una especie de ave urbana optimizan la transmisión acústica en áreas urbanas ruidosas]. Ornitologia Neotropical, 2024, 35(2): 80-86. DOI:10.58843/ornneo.v35i2.1363 |
| 7. | Cueva, L., Bartolo, R., Sandoval, L. The Acoustic adaptation hypothesis does not support the occurrence of common songs in a neotropical urban bird species. Bioacoustics, 2024, 33(6): 510-526. DOI:10.1080/09524622.2024.2422091 |
| 8. | Vargová, V., Balogová, M., Pristašová, P. et al. Spatiotemporal dynamics in the roosting ecology of the green toad: Implications for urban planning and nature conservation. Journal for Nature Conservation, 2024. DOI:10.1016/j.jnc.2023.126543 |
| 9. | Beck, M.L., Sewall, K.B., Akҫay, Ҫ. Experimental manipulation of chest spotting alters territorial aggression in urban and rural song sparrows. Behavioral Ecology and Sociobiology, 2023, 77(12): 136. DOI:10.1007/s00265-023-03396-6 |
| 10. | Simberloff, R.A., Phillips, J.N., Derryberry, G.E. et al. Communication distance predicts territory size: implications for an urban songbird. Animal Behaviour, 2023. DOI:10.1016/j.anbehav.2023.05.016 |
| 11. | Khan, I.A., Kumar, A., Bhatt, D. et al. Tree cover and built-up area regulate the territory size in Eurasian Magpie Pica pica in Ladakh, India. Journal of Threatened Taxa, 2023, 15(7): 23521-23528. DOI:10.11609/jott.8214.15.7.23521-23528 |
| 12. | Lane, S.J., VanDiest, I.J., Brewer, V.N. et al. Indirect effects of urbanization: consequences of increased aggression in an urban male songbird for mates and offspring. Frontiers in Ecology and Evolution, 2023. DOI:10.3389/fevo.2023.1234562 |
| 13. | Rodríguez-Bardía, M., Fuchs, E.J., Barrantes, G. et al. Genetic structure in neotropical birds with different tolerance to urbanization. Scientific Reports, 2022, 12(1): 6054. DOI:10.1038/s41598-022-09961-9 |
| 14. | Hope, S.F., Hopkins, W.A., Angelier, F. Parenting in the city: effects of urbanization on incubation behaviour and egg temperature in great tits, Parus major. Animal Behaviour, 2022. DOI:10.1016/j.anbehav.2022.09.004 |
| 15. | Amorim, P.S., Guaraldo, A.C., Diniz, P. Horneros consider their neighbors as precious foes regardless of territory size and human disturbance. Behavioural Processes, 2022. DOI:10.1016/j.beproc.2023.104942 |
| 16. | Lane, S.J., Sewall, K.B. What about Females? Urban Female Song Sparrows Elevate Aggressive Signaling Compared to Rural. Integrative and Comparative Biology, 2022, 62(3): 487-495. DOI:10.1093/icb/icac106 |
| 17. | Goodchild, C.G., VanDiest, I., Lane, S.J. et al. Variation in Hematological Indices, Oxidative Stress, and Immune Function Among Male Song Sparrows From Rural and Low-Density Urban Habitats. Frontiers in Ecology and Evolution, 2022. DOI:10.3389/fevo.2022.817864 |
| 18. | Juárez, R., Ruiz-Gutiérrez, V., Sandoval, L. Surviving in cities: The case of a year-round territorial bird in the Neotropics. Journal of Urban Ecology, 2022, 8(1): juac006. DOI:10.1093/jue/juac006 |
| 19. | Hamzaj, T., Graham, B., Bonilla, K. et al. Intruder familiarity and not duet similarity influences the territory defense in a year-round territorial bird species. Avian Research, 2022. DOI:10.1016/j.avrs.2022.100032 |
| 20. | Chen, X., Zhang, Q., Lan, S. et al. Nest predation pressure on Chinese Bulbuls decreases along the urbanization gradient in Hangzhou, China. Avian Research, 2022. DOI:10.1016/j.avrs.2022.100049 |
| 21. | Juárez, R., Angulo Irola, M.P., Carman, E.M. et al. Territory size, population density, and natural history of Cabanis’s Ground Sparrow, an endemic species found in urban areas. Ornithology Research, 2021, 29(4): 227-239. DOI:10.1007/s43388-021-00076-9 |
| 22. | Wang, L., Nabi, G., Yin, L. et al. Birds and plastic pollution: recent advances. Avian Research, 2021, 12(1): 59. DOI:10.1186/s40657-021-00293-2 |
| 23. | Diniz, P., Duca, C. Anthropogenic noise, song, and territorial aggression in southern house wrens. Journal of Avian Biology, 2021, 52(10): e02846. DOI:10.1111/jav.02846 |
| 24. | Brunner, N., Kühleitner, M., Renner-Martin, K. Bertalanffy-Pütter models for avian growth. Plos One, 2021, 16(4 April): e0250515. DOI:10.1371/journal.pone.0250515 |
| 25. | Méndez, C., Barrantes, G., Sandoval, L. The effect of noise variation over time and between populations on the fine spectrotemporal characteristics of different vocalization types. Behavioural Processes, 2021. DOI:10.1016/j.beproc.2020.104282 |