| Citation: | Juyong Li, Jing-An Liu, Limin Wang, Dongming Li. 2024: Avian hippocampus: Recent advances in anatomy and physiological functions. Avian Research, 15(1): 100208. DOI: 10.1016/j.avrs.2024.100208 |
The avian hippocampus, akin to its mammalian counterpart, plays a critical role in cognitive and physiological processes despite notable structural differences. Initially thought to be less developed, recent studies over the past two decades have revealed it as a complex brain region essential for diverse functions in both laboratory and free-living birds. This review synthesizes current knowledge on the avian hippocampus' organization, functionality, and neurophysiological significance. We first examine its anatomical structure and neuronal connectivity, comparing it with the mammalian hippocampus. We then highlight how its volume, neuronal density, and neurogenesis support spatial memory and navigation, influencing behaviors such as migration, food storing, brood parasitism, and homing. Beyond spatial functions, the avian hippocampus mediates emotion and stress physiology through interactions with the endocrine system, particularly via glucocorticoid receptors. It also influences spatial memory through sex hormones, especially estradiol, with local estrogen production through aromatase activity enhancing memory and plasticity. Therefore, the avian hippocampus serves as a central neural hub, integrating sensory information with internal states to facilitate essential behaviors and responses to external environmental stimuli. This review underscores the progress made in understanding this brain structure's roles, highlighting conserved neurophysiological functions across vertebrate taxa.
The hippocampus is a major component of the vertebrate brain, forming a crucial part of the limbic system. In mammals, the hippocampus has long captivated researchers due to its critical roles in foundational neurobiological processes such as learning, memory, spatial navigation, anxiety, and stress. It stands as one of the most thoroughly investigated structures in the brain (Knierim, 2015; Madison et al., 2024). Notably, some of its functions are highly conserved across vertebrate taxa (Striedter, 2016; Witter et al., 2017).
In birds, the hippocampus (more specifically the hippocampal formation) mirrors the functional roles of its mammalian counterpart, despite dramatic structural differences. Birds exhibit remarkable cognitive abilities and complex behaviors such as migration, vocal learning, extraordinary memory, and tool use, often rivaling or even surpassing those of mammals (Madison et al., 2024). These fascinating behaviors are supported by fundamental neural functions that may require specialized hippocampal adaptations. Thus, the avian hippocampus serves as an ideal model for studying the structure and evolutionary function of hippocampal-like structures. Comparing the hippocampus between birds and mammals can elucidate the relationship between hippocampal structure and function from the perspectives of comparative neuroanatomy and neural circuitry, offering insights into the evolution and transformation of this brain region over time (Madison et al., 2024).
Early investigations, often comparing birds to mammals, suggested a simplified hippocampal structure in birds, leading to the perception of a less developed system. However, this view has been fundamentally challenged by a surge in studies on the hippocampus of both laboratory avian models and free-living birds (Ben-Tov and Gutfreund, 2022; Gagliardo and Bingman, 2024; Madison et al., 2024). These studies, driven by technological advancements and innovative research approaches, have provided valuable insights into the specific roles of the avian hippocampus, highlighting the evolutionary and functional parallels between avian and mammalian brains. This review synthesizes current knowledge on the complex organization, diverse functionality, and evolutionary significance of the avian hippocampus, reflecting the exciting progress made in understanding this critical brain structure.
In vertebrates, the overall organization of the hippocampal formation is highly conserved. Despite their diverged evolutionary paths, both mammals and birds exhibit homologous structures characterized by the presence of pyramidal-like principal cells, interneurons, and molecular markers (Herold et al., 2015). While birds and mammals have independently evolved over 200 million years (Striedter, 2016), the specific structures within this homologous area manifest notable differences between the two classes (Morandi-Raikova and Mayer, 2022).
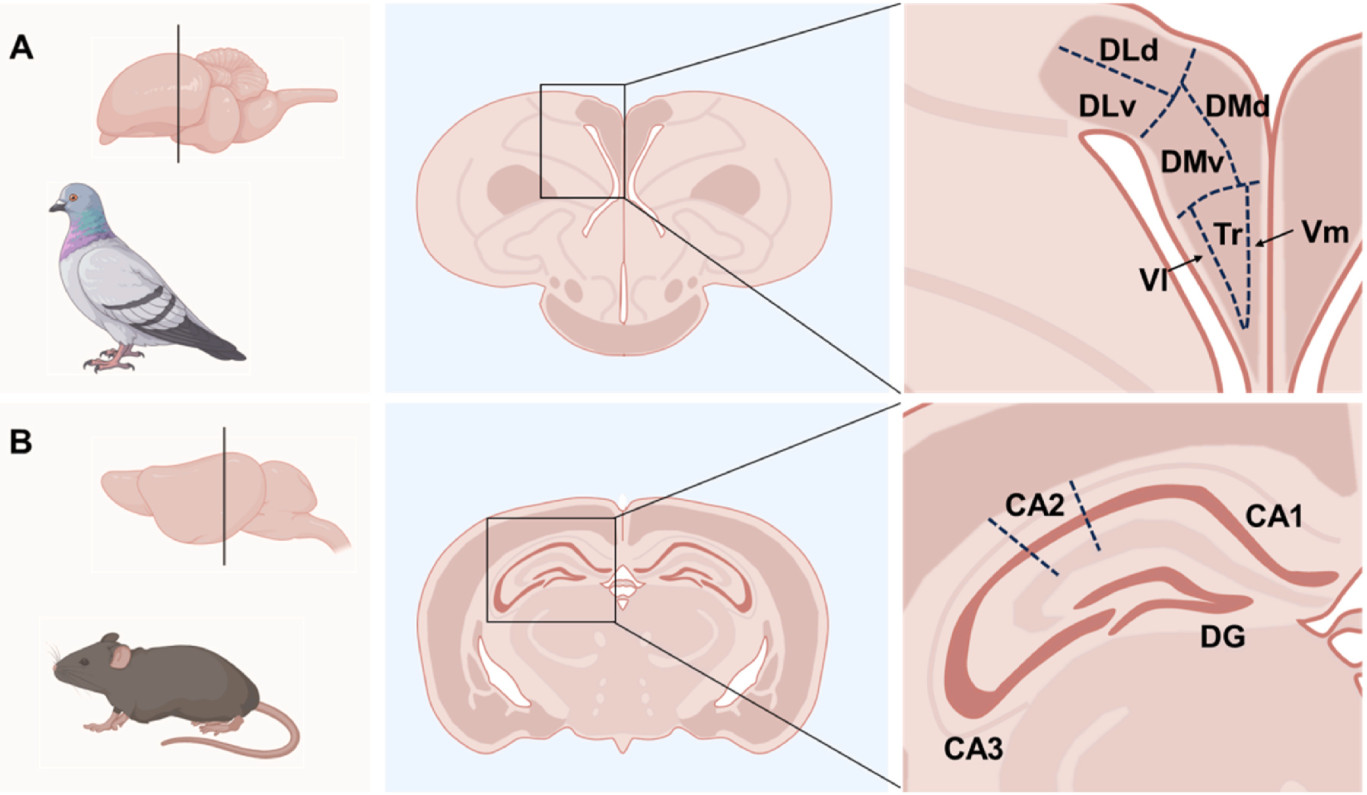
The mammalian hippocampus is located within the medial temporal lobe and typically features a three-layered organization. This structure is subdivided along the transverse axis into distinct regions: the dentate gyrus (DG), the CA fields (CA, referring to “cornu ammonis”, or Ammon's horn, which are further divided into CA1, CA2, and CA3 based on the density and size of pyramidal neurons), and the subiculum (Fig. 1; El-Falougy and Benuska, 2006). In contrast, the avian hippocampus (referred to as the hyperpallium apicale) is situated in the dorsomedial section of the telencephalon, positioned in the posterior medial area of the pallial hemisphere, just below the dorsal surface and above the lateral ventricle (Atoji and Wild, 2006; Chen et al., 2013; Herold et al., 2015).
Unlike the layered organization of the mammalian hippocampus, the avian hippocampal area comprises a densely packed and diverse array of neuron types that are distributed homogeneously, often forming clusters or sparse networks rather than laminae (Atoji and Wild, 2004; Atoji et al., 2016). This results in an architecture distinct from that of mammals (Herold et al., 2015, 2019; Stacho et al., 2020). The avian hippocampus does not have clear boundaries from adjacent telencephalic structures (Herold et al., 2014; Striedter, 2016), leading to variability in the proposed subdivisions, their boundaries, and nomenclature, especially concerning phylogeny.
Within species, the nomenclature and proposed number of subdivisions are subjects of ongoing debate. In Pigeons (Columba livia domesticus), Karten and Hodos (1967) initially described two main subdivisions: a hippocampus proper (Hp) and an area parahippocampalis (APH), based on Nissl stains (Karten and Hodos, 1967). Erichsen et al. (1991) later identified five subdivisions: a dorsally extending medial fiber tract, a dorsomedial area, a V-shaped area of large cells, a ventral region, and the inferior part of the dorsomedial region (Erichsen et al., 1991). In Zebra Finches (Taeniopygia guttata), Szekely and Krebs (1996) identified three subdivisions: the dorsolateral (DL), dorsomedial (DM), and ventral (V) regions (Szekely and Krebs, 1996), whereas Montagnese et al. (1996) recognized five subdivisions, including the APH, lateral and medial hippocampal layers, the central field of the APH, and a crescent field (Montagnese et al., 1996). In Chickens (Gallus gallus domesticus), the hippocampus has been described as consisting of five subdivisions: APHl, APHi, APHre, APHm, and Hp (Puelles et al., 2007). Gene expression pattern analysis further revealed a different scheme: a ventral V-shaped area and the DM and DL regions. The chicken hippocampus is comparable to the pigeons, although it does not fully align with the previously described DM and DL (Gupta et al., 2012). This lack of consensus in the anatomical subdivisions of the avian hippocampus presents a challenge for researchers interested in this brain region.
Currently, there is general agreement that the avian hippocampus can be divided into a DL, a DM, and a ventral V-shaped area (Atoji and Wild, 2004, 2006; Gupta et al., 2012; Herold et al., 2014; Atoji et al., 2016). The DL can be further subdivided into dorsal (DLd) and ventral (DLv) components based on connectivity and receptor architecture (Atoji and Wild, 2004; Herold et al., 2014). The DM can also be divided into dorsal (DMd) and ventral (DMv) components, distinguished by hippocampal receptor densities (Herold et al., 2014). The V-shaped area consists of a triangular region (Tr) surrounded by a thin V-shaped layer, which is clearly visible due to its higher neuron density (Atoji and Wild, 2004). This V-shaped layer includes adjacent ventromedial (Vm) and ventrolateral (Vl) dense cell layers (Fig. 1).
While the avian hippocampus differs structurally and neurally from the mammalian hippocampus, its functional role and connectivity patterns with other brain regions appear highly conserved across taxa (Sherry and Hoshooley, 2010; Herold et al., 2015). In mammals, the DG receives major inputs from the entorhinal cortex (EC) (Chawla et al., 2005; Danielson et al., 2016). A DG homolog has been identified in the ventral region of the hippocampus in both pigeons and chickens (Atoji and Wild, 2004; Gupta et al., 2012). Combining tract-tracing and gene expression studies, the avian DM has been shown to be homologous to the mammalian Ammon's Horn (CA3/CA1), while the dense cell layers VM and VL are homologous to the mammalian DG (Atoji et al., 2016).
In pigeons, receptor-binding analyses identified 11 neurotransmitter receptors and found similarities between the Vl/Tr/DMv regions and the mammalian DG/CA1, with some evidence that DMd/Vm might be comparable to CA2/CA3. Other studies have suggested that avian DLd and DLv are analogous to the entorhinal cortex (Atoji and Wild, 2004; Rattenborg et al., 2011). The DMd shared receptor characteristics with CA3, while the DMv was generally more similar to CA1 and Vm to CA2 (Fig. 2). The rostral portion of the avian hippocampus has been proposed as equivalent to the rodent dorsal hippocampus, with the caudal portion analogous to the rodent ventral hippocampus (Smulders, 2017, 2021), suggesting that these regions may perform similar functions to their mammalian counterparts (O'Leary and Cryan, 2014; Gualtieri et al., 2019; Herold et al., 2019; Armstrong et al., 2020a). Functionally, the avian caudal hippocampus is more sensitive to stress-induced decreases in adult neurogenesis (Gualtieri et al., 2019; Herold et al., 2019; Armstrong et al., 2020a), analogous to the ventral hippocampus of mammals (O'Leary and Cryan, 2014). These findings have provided valuable insights for investigating possible correspondences between avian and mammalian hippocampal subdivisions (Herold et al., 2014).
The mammalian hippocampus is characterized by complex connectivity among its regions. The EC serves as the primary input structure, linking the hippocampus to the rest of the neocortex (Kerr et al., 2007). Within the hippocampus, information flows primarily along the trisynaptic pathway: the DG receives input from the EC, which is then relayed to CA3, CA1, and back to the EC (Treves et al., 2008; Danielson et al., 2016). In birds, a similar feed-forward pathway exists within the hippocampal formation, mirroring the mammalian trisynaptic pathway (Kahn et al., 2003). For instance, in pigeons, information flow starts in the DL region, passes to the DM region, then to the Vm, onward to the Vl, and finally back to DM, which projects out of the hippocampus (Kahn et al., 2003). Moreover, the DM is reciprocally connected with both the Tr and DL, and DL has similar bidirectional connections with DM and Tr.
Recent research identified two distinct hippocampal pathways in birds, a superficial dorsomedial pathway and a deep ventrolateral pathway, along the hippocampal transverse axis (Rook et al., 2023). In the superficial pathway, DLd projects to DMd and medial Tr, and to a lesser extent Vm. DMd projects to Vm and medial Tr, and back to DLd, as well as having reciprocal connections with DMv. Vm then projects to Vl and Tr. Meanwhile, in the deep pathway, DLv primarily projects to DMv, with weaker connections to Vl. From DMv, efferents reach Vl and lateral Tr. Vl sends projections back to DMv and Tr. Projections to DMd and DMv originate from medial and lateral Tr, respectively. These parallel pathways interconnect at DL and DM, as well as via the V-shaped area through connections between lateral and medial Tr (Fig. 3A and B) (Rook et al., 2023).
The avian hippocampus receives extrinsic inputs from various sensory pathways. Visual centers like the Wulst provide spatially mediated associative learning inputs (Atoji and Wild, 2019). The hyperpallium, analogous to parts of the mammalian neocortex, offers processed sensory information (Reiner et al., 2004). Other sources include the nidopallium for somatosensory and auditory inputs, and the dorsolateral corticoid area receiving olfactory inputs, along with projections from numerous other brain regions (Atoji and Wild, 2004). Additional inputs come from pallial and thalamic areas involved in sensory processing and geomagnetic navigation as well as emotional responses, such as the medial ventral arcopallium (AMV, analogous to mammalian amygdala) (Mello et al., 2019; Heyers et al., 2022). Notably, in songbirds, the rostral hippocampus receives more thalamic inputs, while the caudal hippocampus receives more from AMV, indicating a functional organization along the long axis (Applegate et al., 2023).
In pigeons, efferent projections primarily arise from the DM and DL divisions, reciprocating major afferents (Fig. 3C). The DM projects to other pallial regions, septal areas, and hypothalamic areas, including indirect connections to the paraventricular nucleus (PVN) (Atoji and Wild, 2004; Smulders, 2021). Subsequent studies have revealed even more diverse connection pathways (Atoji et al., 2016). Despite anatomical differences, significant homologies exist between avian and mammalian hippocampal structures, both of which are pallial in origin (Reiner et al., 2004; Jarvis et al., 2005). Similar to mammals, avian hippocampal subdivisions communicate chemically (Atoji and Wild, 2006; Herold et al., 2015). Neuropeptides such as substance P, neuropeptide Y, somatostatin, cholecystokinin, and vasoactive intestinal polypeptide, along with neurotransmitters like glutamate, found in the mammalian hippocampus, are also present in the avian hippocampus (Rosinha et al., 2009). These features underscore the avian hippocampus' role in regulating diverse physiological functions, including learning, memory, spatial navigation, visual discrimination, and emotional and sexual behavior.
Since the discovery of hippocampal “place cells” in mammals (O'Keefe and Dostrovsky, 1971) and the development of the hippocampus as a cognitive map theory, along with studies on the impact of mammalian hippocampal lesions on place navigation (Morris et al., 1982), research has increasingly explored the role of the avian hippocampus in spatial memory and navigation (Fig. 4, Table 1).
| Function | Order | Family | Species | Treatment, stimulus, or measurement | Main points | References |
| Spatial memory | Passeriformes | Paridae | Black-capped Chickadee (Poecile atricapillus) | Food-caching, neuronal acitivity in hippocampus | An increase of the number of immediate early genes immunoreactive neurons within hippocampus after food hoarding. | Smulders and DeVoogd, 2000; Bailey et al., 2002; Ben-Tov and Gutfreund, 2022 |
| Hippocampal lesion | Disrupted spatial memory in food-storing birds. | Patel et al., 1997 | ||||
| Food-caching, hippocampal lesion | Disrupted birds' ability to recall the locations of stored or hidden seeds but do not impair the motivation to store. | Sherry et al., 1989 | ||||
| Food-caching, hippocampus volume | Food-caching species had bigger hippocampus relative to non-storing species. | Krebs et al., 1989; Healy and Krebs, 1992 | ||||
| Food-caching, neuronal density in hippocampus | Experience of food storing and retrieval resulted in hippocampus enlargement with an increase in neuronal number. | Clayton and Krebs, 1994 | ||||
| Tufted Titmouse (Baeolophus bicolor) | Food-caching | Accurate cache retrieval requires the hippocampus, food-caching birds are memory specialists, capable of remembering many scattered, concealed food locations. | Payne et al., 2021 | |||
| Icteridae | Brown-headed Cowbird (Molothrus ater) | Brood parasitism, hippocampus volume and hippocampal neurogenesis | Females, not males, remember locations of various potential host nests to monitor progress of nest-building and egg-laying. | Guigueno and Sherry, 2017 | ||
| Brood parasitism, hippocampus volume | Females performed better than males on a spatial task and had a larger hippocampus than males. | Sherry et al., 1993; Guigueno et al., 2014 | ||||
| Shiny Cowbird (Molothrus bonariensis) | Hippocampus volume | No sex differences were found in Screaming Cowbirds, a brood parasite in which males assist females in nest searching. | Reboreda et al., 1996 | |||
| Brood parasitism, hippocampus volume | Females had bigger hippocampus size than males, due to only females searching for nests. | Reboreda et al., 1996 | ||||
| Emberizidae | White-crowned Sparrow (Zonotrichia leucophrys) | Hippocampus volume and hippocampal neurogenesis | Migratory sub-species have a larger hippocampus and greater hippocampal neurogenesis than non-migratory sub-species. | Pravosudov et al., 2006; LaDage et al., 2011 | ||
| Sylviidae | Reed Warbler (Acrocephalus scirpaceus) | Hippocampal neurogenesis | Hippocampal neurogenesis is greater in a migratory Reed Warbler than their closely related non-migratory species. | Barkan et al., 2014 | ||
| Hirundinidae | Purple Martin (Progne subis) | Hippocampus volume and hippocampal neurogenesis | Hippocampus size and neurogenesis are closely associated with patterns of space use. | Lalla et al., 2022 | ||
| Estrildidae | Zebra Finch (Taeniopygia guttata) | Acquisition and retention of spatial learning | The hippocampus is involved in the storage as well as in the retrieval of spatial memory. | Watanabe and Bischof, 2002, 2004; Bischof et al., 2006; Mayer et al., 2010 | ||
| Acquisition and retention of spatial learning | Expression of immediate early genes (c-fos and ZENK) is up-regulated in hippocampus during spatial task learning and recall. | Bischof et al., 2006; Mayer et al., 2010 | ||||
| Hippocampal lesion, acquisition and retention of spatial learning | Affected the acquisition and retention of spatial learning tasks and lead to deficits in spatial memory. | Watanabe and Bischof, 2002, Watanabe and Bischof, 2004; Bischof et al., 2006 | ||||
| Acute stress | High CORT response induced by acute stress impaired spatial learning and memory. | Hodgson et al., 2007 | ||||
| Inhibiting hippocampal estrogens | Inhibiting hippocampal estrogens by antagonizing aromatase with ATD (1,4,6-adrostatriene-2,17-dione), caused worse spatial memory. | Bailey et al., 2013 | ||||
| Charadriiformes | Scoiopacidae | Semipalmated Sandpiper (Calidris pusilla) | Hippocampus volume | Hippocampus volume was larger in wintering birds than migrating birds. | de Morais Magalhaes et al., 2017 | |
| Spatial navigation | Columbiformes | Columbidae | Homing Pigeon (Columba livia) | Hippocampus volume | Those individuals with developmental experience navigating to a home loft had larger hippocampi than those that did not. | Cnotka et al., 2008 |
| Hippocampal lesion | Caused a profound loss of navigational abilities. | Colombo et al., 2001 | ||||
| Hippocampal lesion | Lost their abilities to orient their vanishing bearings towards home from a familiar training site. Impaired performance before learning, but not when maps were already acquired. | Gagliardo et al., 2009; Gagliardo, 2013; Coppola et al., 2014b | ||||
| Hippocampal activity | Upregulated hippocampal neuronal activation when they homed from a familiar location. | Shimizu et al., 2004 | ||||
| Hippocampal lesion | Impaired spatial learning behavior in a radial maze task, and performed poorly on spatial autodiscrimination task. | Watanabe, 1999; Colombo et al., 2001 | ||||
| Galliformes | Phasianidae | Domestic Chicken (Gallus gallus) | Spatial encoding of goal location | The hippocampus is instrumental for enabling the spatial encoding of goal locations. | Tommasi et al., 2003; Morandi-Raikova and Mayer, 2021 | |
| Visual-spatial perception | Columbiformes | Columbidae | Homing Pigeon (Columba livia) | Hippocampal lesion | Take a straighter path home compared to controls. | Gagliardo et al., 2014; Herold et al., 2015 |
| Hippocampal lesion | More likely to display high frequency, oscillatory shifts in their flight paths. | Gagliardo et al., 2023 |
Spatial cognition, crucial for behaviors like migration, foraging, and homing, allows animals to perceive environmental information to support complex decision-making, which is vital for survival (Milford and Schulz, 2014). Birds exhibit exceptional spatial memory and navigational abilities (Buschman and Miller, 2014). Specific hippocampal neurons are finely tuned to process and encode environmental information, making the hippocampus essential for flexible navigation (Wirt and Hyman, 2017; Li et al., 2020). For instance, Bingman et al. (1984) showed that pigeons with hippocampal lesions had difficulty using familiar landmarks to navigate. Similarly, Sherry et al. (1989) found that hippocampal lesions disrupted food-storing Black-capped Chickadees' (Poecile atricapillus) ability to recall stored seed locations. Over the past two decades, many studies have highlighted the avian hippocampus' role in regulating spatial memory across species (Smulders, 2006; Salwiczek et al., 2010; Sherry and Hoshooley, 2010), showing it supports the spatial encoding of goal locations in species like Zebra Finches (Mayer et al., 2010; Mayer and Bischof, 2012), homing pigeons (Bingman et al., 2006), and chickens (Morandi-Raikova and Mayer, 2021). These studies affirm the hippocampus’ connection to spatial memory conservation and decision-making (Olafsdottir et al., 2018).
Research into the spatial response properties of hippocampal neurons highlights the critical role of the hippocampus in spatial learning and memory. In pigeons, hippocampal neurons show spatially modulated responses distinct from mammalian “place cells” (Siegel et al., 2006; Kahn et al., 2008), but resembling spatial responses when tested with stable goal locations (Siegel et al., 2006). Similarly, spatial response properties in rats are influenced by stable goal locations (Sosa and Giocomo, 2021). Studying Zebra Finches and Tufted Titmice (Baeolophus bicolor), Payne et al. (2021) also recorded place cell-like activity, which was more pronounced in food-storing titmice than in non-storing Zebra Finches (Payne et al., 2021).
The hippocampal-dependent memory system varies significantly between food-storing and non-food storing species (Hampton and Shettleworth, 1996). The relative hippocampus size is positively related to food-storing behavior (Lucas et al., 2004), e.g., food-storing birds belonging to Paridae, Sittidae, and Corvidae families have a larger relative hippocampus size than those from non-food-storing counterparts (Healy and Krebs, 1992). Those food-storing species with better memory of storing and retrieval experience results in enlargement of hippocampus with an increase in neuronal number relative to their closely related species, e.g., in food-storing Marsh Tit (Parus palustris) relative to Blue Tit (P. caeruleus), and food-storing Jay (Garrulus glandarius) relative to Jackdaw (Corvus monedula; Clayton and Krebs, 1994). Seasonal changes in hippocampal volume reflect fluctuations in memory processing associated with food-storing and retrieval (Ben-Tov and Gutfreund, 2022; Lange et al., 2022). Notably, food-storing species show an increase in hippocampal neuron numbers and neurogenesis during the fall, which is the primary season for food-storing (Sherry and Hoshooley, 2010). During this period, the heightened expression of immediate early genes (IEGs), which serve as neuronal activity markers, in the hippocampus highlights the activation associated with spatial map acquisition (Smulders and DeVoogd, 2000). Crucially, hippocampal lesions have been shown to disrupt spatial memory in food-storing Black-capped Chickadees (Hampton and Shettleworth, 1996).
Non-food-storing birds, though they do not travel long distances, possess remarkable spatial memory, allowing them to recognize territorial boundaries and locate essential resources such as food, water, and nesting sites. For instance, Zebra Finches, a non-storing songbird, have the ability to use a spatial map for finding food (Watanabe and Bischof, 2004; Bischof et al., 2006; Mayer et al., 2010). Hippocampal lesions in Zebra Finches disrupt their spatial memory, resulting in more frequent visits before locating the correct feeder (Watanabe and Bischof, 2004). Additionally, other non-food-storing birds, such as pigeons, require strong spatial learning and memory when searching for food and homing (Herold et al., 2019; Rook et al., 2023). These conditions may necessitate larger hippocampal volumes to enhance their spatial memory capabilities, enabling them to adapt and survive during challenging times.
Brood parasitism heavily relies on spatial memory, as brood-parasitic birds must locate and remember heterospecific nests to lay their eggs successfully, a task primarily undertaken by the female. In typical brood parasite species like the Brown-headed Cowbird (Molothrus ater), females display more complex spatial use and better spatial memory than males, as they visit numerous host nests throughout the breeding season to evaluate and manipulate them (Guigueno et al., 2014). Females also have a larger hippocampus relative to the telencephalon compared to males (Sherry et al., 1993). This sex difference is observed in Shiny Cowbirds (M. bonariensis) as well, where only females search for nests (Reboreda et al., 1996). However, no such difference exists in Screaming Cowbirds (M. rufoaxillaris), where males assist females with nest searching (Reboreda et al., 1996).
Collectively, these variations mentioned above in the hippocampal memory systems across different lifestyles suggest that a complex and larger hippocampal structure is crucial for maximizing fitness. This development is driven by the selective pressures to enhance spatial memory capabilities.
Birds demonstrate a wide range of navigational movements, from local routes covering tens of meters to thousands of kilometers-long migratory journeys (Bingman and Cheng, 2005). The avian hippocampus is crucial in navigating these spatial tasks, helping birds calculate and execute paths from one location to another.
In homing pigeons, the ability to return to their loft from distant, unfamiliar locations is particularly noteworthy. The hippocampus is vital for navigation based on familiar landmarks, forming a memory representation similar to a cognitive map (Herold et al., 2015). Studies show that pigeons with developmental experience in homing have larger hippocampus (Cnotka et al., 2008), and damage to this region results in significant navigational deficits (Colombo et al., 2001). Pigeons with hippocampal lesions struggle to navigate using familiar landmarks, failing to orient themselves towards home from familiar sites (Coppola et al., 2014). This damage also impairs spatial learning tasks and decreases performance in spatial discrimination tasks (Watanabe, 1999).
Migratory birds rely on remembering environmental cues during their seasonal journeys. Research indicates that migratory birds have larger hippocampi compared to non-migratory species (Bingman and MacDougall-Shackleton, 2017). For example, migratory Reed Warblers (Acrocephalus scirpaceus) exhibit greater hippocampal neurogenesis compared to the closely related non-migratory Clamorous Warblers (A. stentoreus; Barkan et al., 2014). Intraspecifically, migratory sub-species of White-crowned Sparrows (Zonotrichia leucophrys) show greater hippocampal size and neurogenesis than non-migratory ones (LaDage et al., 2011). In Semipalmated Sandpipers (Calidris pusilla), a long-distance migrant, hippocampal size varies seasonally, being larger in winter after migration (de Morais Magalhaes et al., 2017). However, the exact role of the hippocampus in long-distance migration remains unclear (Bingman and MacDougall-Shackleton, 2017).
Navigation, as a complex cognitive process, heavily depends on encoding spatial information from the environment, involving multiple brain regions working together (Mouritsen et al., 2016). The interaction between the hippocampus and other brain areas enables tasks like spatial memory, navigation decision-making, and path adjustment (Goodroe et al., 2018; Ito, 2018). In mammals, hippocampal neurons can receive direct input from the prefrontal cortex (PFC) (Cholvin et al., 2018), and lesions in the hippocampus and PFC lead to reduced spatial learning performance (Tuscher et al., 2018; Zielinski et al., 2019; Avigan et al., 2020). This relationship provides a physiological basis for the anatomical and functional connections between these regions. Similarly, in birds, the hippocampus is responsible for encoding the current and goal locations, while the nidopallium caudale (NCL) focuses on routing information related to goal-directed decision-making (Chen et al., 2018). Damage to the hippocampus and NCL in pigeons impairs spatial navigation tasks (Gagliardo et al., 2009). Spatial cognition and navigation information are first perceived and stored in the hippocampus, then transmitted to the NCL for further processing (Li et al., 2020). This coordinated interaction supports different functions in navigation, emphasizing the roles of both the hippocampus and NCL in complex spatial tasks (Taufique et al., 2018a; Li et al., 2020).
The avian hippocampus plays a dual role in both recognizing the location of a goal and navigating towards it, relying heavily on visual perception and spatial memory. Recent advancements in tracking technology, such as global positioning system (GPS)-recording devices, have been used to study the visual-spatial perception of hippocampal-lesioned birds (Gagliardo and Bingman, 2024). These studies show that hippocampal-lesioned homing pigeons, when flying home from unfamiliar locations, tend to take straighter paths than control pigeons. This behavior suggests a deficit in visual-spatial perception or attention (Gagliardo et al., 2014; Herold et al., 2014).
Further research found that these lesioned pigeons showed lower route fidelity when repeatedly flying home from the same locations, indicating difficulties in utilizing visual landmarks and landscape features for memory and navigation (Gagliardo and Bingman, 2024). This was evidenced by high-frequency, oscillatory shifts in their flight paths, suggesting they were seeking visual information (Gagliardo et al., 2023). In contrast, control pigeons maintained route fidelity by following landscape features such as roads and woodland margins. The loss of path fidelity in hippocampal-lesioned pigeons is thought to be due to perceptual or attentional impairments, particularly in recognizing landscape boundaries.
During familiar navigation, particularly around their home area, homing pigeons utilize a secondary map-like mechanism based on visual landmarks (Biro et al., 2004; Guilford and Biro, 2014). Elevated neuronal activation in the hippocampus is observed when pigeons home from familiar locations (Shimizu et al., 2004). In the local navigation phase, as pigeons approach within kilometers of their loft, they switch to relying on familiar visual landscape features (Gagliardo et al., 2007).
Overall, the cumulative findings from neuroanatomy, neurochemistry, neuronal spatial response properties, and lesion studies suggest that the avian hippocampus has a functional profile that extends beyond merely providing a neutral, cognitive map-like representation of space.
Beyond its roles in memory and spatial navigation, the avian hippocampus is also active in response to stress and anxiety, offering insights into its functions “beyond space.” In mammals, the hippocampus is closely linked to anxiety induced by stress (Shimizu et al., 2004; Smulders, 2017; Larosa and Wong, 2022). Chronic stress can lead to hippocampal atrophy in humans, often due to glucocorticoid dysregulation (Sapolsky, 2000). Similarly, the avian hippocampus is crucial for managing various stress responses to internal and external stimuli, such as physical conditions, social hierarchy, food availability, and environmental changes (Fig. 4, Table 2).
| Function | Order | Family | Species | Treatment, stimulus, or measurement | Main points | References |
| Anxiety-related behavior | Columbiformes | Columbidae | Homing Pigeon (Columba livia) | Hippocampal lesion, approach-avoidance conflict | Ignore the presence of a human in the testing room. | Broadbent and Colombo, 2000 |
| Galliformes | Phasianidae | Domestic Chicken (Gallus gallus) | Long tonic immobility | Laying hens had higher expression of proliferating cell nuclear antigen in hippocampus. | Armstrong et al., 2020b | |
| Approach-avoidance conflict, passive avoidance learning test | Those learned to avoid a bitter event (the methyl anthranilate bead) showed reduced number of newly-generated cells in hippocampus. | Nikolakopoulou et al., 2006 | ||||
| Japanese Quail (Coturnix japonica) | Social isolation stress | Exhibited c-Fos reactivity especially in the dorsolateral area of hippocampus. | Takeuchi et al., 1996 | |||
| Long tonic immobility | Fewer newly-generated cells in hippocampus. | Lormant et al., 2020 | ||||
| Passeriformes | Passeridae | House Sparrow (Passer domesticus) | Approach-avoidance conflict, neophobic behavior | Increased with those gene expressions of caudal hippocampus, i.e., increased neuronal activity in response to novel objects. | Lattin et al., 2022; Kimball et al., 2022 | |
| Stress-related physiology | Passeriformes | Paridae | Black-capped Chickadee (Poecile atricapillus) | Captivity | Reduced hippocampal volume. | Tarr et al., 2009 |
| Captivity | Reduced hippocampal neurogenesis. | Barnea and Nottebohm, 1994 | ||||
| Mountain Chickadee (Poecile gambeli) | Social ranking | Subordinate individuals had lower levels of cell proliferation in the hippocampus than dominant birds. | Pravosudov and Omanska, 2005 | |||
| Captivity | Captive individuals have less newly-generated neurons relative to those wild individuals. | LaDage et al., 2010 | ||||
| CORT administration | Showed superior cache retrieval and spatial memory performance compared with control birds. | Saldanha et al., 2000; Pravosudov, 2003 | ||||
| Unpredictable food availability | Small but chronic elevations in CORT triggered correlated with enhanced cache retrieval and performance. | Pravosudov and Clayton, 2001 | ||||
| Icteridae | Brown-headed Cowbird (Molothrus ater) | Captivity | Reduced hippocampal volume. | Day et al., 2008 | ||
| Emberizidae | White-crowned Sparrow (Zonotrichia leucophrys) | Acute stress | Nt changes either MR or GR expression in the hippocampus. | Krause et al., 2021 | ||
| Estrildidae | Zebra Finch (Taeniopygia guttata) | Dim light at night | Increased proliferation in the ventricular zone adjacent to hippocampus, and increased recruitment and total neuron numbers in hippocampus. | Moaraf et al., 2020a, Moaraf et al., 2020b, Moaraf et al., 2021 | ||
| Domestic Chicken (Gallus gallus) | Chronic food restriction | Reduced the number of newly-generated neurons in hippocampus. | Robertson et al., 2017 | |||
| Corvidae | Indian House Crow (Corvus splendens) | Constant light | Decreased DCX + neurons, smaller neuronal somata, fewer glia in hippocampus. | Taufique et al., 2018a, Taufique et al., 2019 | ||
| Dim light at night | Decreased the expression of several hippocampal genes including BDNF. | Taufique et al., 2018b | ||||
| Passeridae | House Sparrow (Passer domesticus) | Captivity | Declined dendrite length and spine density of hippocampal neurons. | Roth 2nd et al., 2017 | ||
| Emberizidae | Dark-eyed Junco (Junco hyemalis) | Captivity | Reduced hippocampal volume. | Smulders et al., 2000 | ||
| Sylviidae | Garden Warblers (Sylvia borin) | Captivity | No difference in hippocampus volume in response to captivity for 5 days or 1 year. | Healy et al., 1996 | ||
| Hirundinidae | Tree Swallow (Tachycineta bicolor) | Extreme environments | Increased MR mRNA expression but not GR in hippocampus. | Zimmer et al., 2023 | ||
| Sturnidae | European Starlings (Sturnus vulgaris) | Chronic stress | Increased MR mRNA expression in hippocampus. | Dickens et al., 2009 | ||
| Galliformes | Phasianidae | Domestic Chicken (Gallus gallus) | Acute (24 h) food deprivation | Reduced spine numbers in hippocampus. | Kumar et al., 2023 | |
| Lariformes | Laridae | Blacklegged Kittiwake (Rissa tridactyla) | CORT administration | Compromised spatial ability during early development. | Kitaysky et al. 2003 | |
| Sexual behavior | Galliformes | Phasianidae | Japanese Quail (Coturnix japonica) | Conditional stimulus of sexual behavior | Exhibited FOS-immunoreactive fibers in hippocampus. | Taziaux et al., 2007 |
| Song learning | Passeriformes | Estrildidae | Zebra Finch (Taeniopygia guttata) | Conspecific or heterospecific song stimuli | Higher density of FOS-immunoreactive cells in hippocampus when they heard conspecific learned song. | Bailey et al., 2002 |
In mice, newly-generated neurons in the ventral DG help inhibit anxiogenic mature granule cells, increasing resilience to anxiety from chronic stress (Anacker et al., 2018). Comparable processes may occur in birds. For instance, Japanese Quails (Coturnix japonica) with prolonged tonic immobility, a sign of high anxiety, had fewer new hippocampal cells than those with shorter immobility (Lormant et al., 2020). Conversely, laying hens with prolonged immobility showed increased cell division in both rostral and caudal hippocampus (Armstrong et al., 2020a). Additional evidence for the hippocampus' response to anxiogenic situations comes from studies on acute and chronic social isolation stress. In Japanese Quail chicks, acute social isolation stress triggered c-Fos reactivity, particularly in the DL area, extending throughout the rostro-caudal axis (Takeuchi et al., 1996). In broiler chicks, social isolation stress induced changes in the hippocampal metabolome, an effect partly replicated by corticosterone (CORT) treatment but not as significantly by heat stress (Brown et al., 2023). These findings underscore the hippocampus' involvement in mediating stress responses and its sensitivity to different types of stressors.
Approach-avoidance conflicts present an anxiogenic situation where animals must weigh potential rewards against possible risks, navigating a delicate balance between seeking positive outcomes and avoiding harm. In mammals, the hippocampus is crucial in mediating these conflicts, helping generate clear “approach” or “avoid” decisions (Loh et al., 2017; Bryant and Barker, 2020). A similar role is observed in birds, where the hippocampus contributes significantly to approach-avoidance decision-making. For instance, pigeons with hippocampal lesions tend to show increased approach behavior; they are more inclined to disregard the presence of a human in the testing environment, suggesting a disruption in their typical avoidance responses (Broadbent and Colombo, 2000). This indicates that the avian hippocampus, like its mammalian counterpart, plays an essential role in processing the complex interplay of motivational and emotional factors involved in these decision-making scenarios.
A similar hippocampal role is noted in feeding neophobia trials (a type of approach-avoidance behavior; Tobler and Sandell, 2007; Schaffer et al., 2021), where birds must resolve conflicting stimuli—food vs. novel objects (Bannerman et al., 2014; O'Neil et al., 2015). In House Sparrows (Passer domesticus), neophobia is linked to caudal hippocampus gene expression, suggesting the region's involvement in approach-avoidance decisions (Kimball et al., 2022; Lattin et al., 2022). These experiments often included spatial elements, with novel objects as unexpected stimuli in familiar contexts, requiring further research to separate the hippocampus' role in approach-avoidance-related behavior from spatial representation (Kimball et al., 2022).
In passive avoidance learning tests, chicks are trained to avoid a negative experience, such as tasting a bitter substance like a methyl anthranilate bead (Lee-Teng and Sherman, 1966). This aversive event triggers activation of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in increased cortisol levels in the hippocampus (Sandi and Rose, 1997). Following such stress-inducing experiences, a decrease in newly-generated hippocampal cells is observed after 24 h (Nikolakopoulou et al., 2006). Interestingly, in contrast to these observations, lesions in the hippocampus tend to lead to decreased anxiety levels, rather than increased anxiety levels (Bannerman et al., 2004). These findings suggest that the avian hippocampus plays a complex role in linking negative emotional information with spatial cues. Similar to its role in mammals, the hippocampus integrates emotional valence with spatial cognition, encoding the emotional significance of experiences within their spatial context (Jin and Lee, 2021). This integration helps guide behavior and decision-making processes, particularly in response to aversive experiences.
Keel bone fractures in commercial laying hens lead to a reduction in doublecortin (DCX, endogenous markers of immature neurons) + neuron density in the hippocampus, particularly in the caudal regions for those with more recent fractures (Armstrong et al., 2020b). Hens in poorer physical condition, often subordinate in their social groups, exhibit lower DCX + densities across both hippocampal poles compared to healthier birds (Armstrong et al., 2022). In Mountain Chickadees (Poecile gambeli), subordinate individuals demonstrate lower cell proliferation levels in the hippocampal ventricular zone than dominant ones (Pravosudov and Omanska, 2005).
Acute (24 h) food deprivation leads to a reduced number of dendritic spines in the hippocampus in chicks (Kumar et al., 2023), and chronic food restriction reduces the number of newly-generated neurons in both hippocampal regions in broiler breeders (Robertson et al., 2017). In wild Indian House Crows (Corvus splendens), constant light exposure reduces DCX + neurons, neuronal size, and glial cell numbers in the hippocampus compared to birds on a regular light-dark cycle (Taufique et al., 2018a, 2019). Additionally, dim light at night decreases the expression of several hippocampal genes, including brain-derived neurotrophic factor (BDNF) (Taufique et al., 2018b). Contrastingly, Zebra Finches exposed to dim light at night show increased neuronal proliferation and recruitment, suggesting species-specific responses to light conditions (Moaraf et al., 2020a, 2020b).
Stress factors associated with captivity, such as spatial confinement, reduced activity, and social isolation, seem to negatively affect the hippocampus, particularly neurogenesis. Bringing wild birds into captivity, a chronic stressor, generally results in reduced hippocampal volume compared to their wild counterparts, as observed in species like Dark-eyed Juncos (Junco hyemalis; Smulders et al., 2000), Brown-headed Cowbirds (Day et al., 2008), Mountain Chickadees (LaDage et al., 2009), Black-capped Chickadees (Tarr et al., 2009), and House Sparrows (Roth et al., 2017). Captivity also leads to a decline in dendrite length and dendritic spine density, with House Sparrows showing increased spine densities when moved from smaller cages to larger aviaries, indicating the relation between spine density and available space (Roth et al., 2017). Similarly, captivity reduces hippocampal neurogenesis in Black-capped Chickadees and Mountain Chickadees when measured using immature neurons marker DCX (LaDage et al., 2010). These alterations in hippocampal volume and spine densities likely stem from less enriched environments in captivity, impacting spatial information processing. Interestingly, Garden Warblers (Sylvia borin), a migratory songbird species, show no change in hippocampal volume when kept in captivity for 5 days or 1 year (Healy et al., 1996). This suggests that changes in hippocampal volume may differ according to phylogeny.
In songbirds, song perception is crucial for reproductive success in both sexes. Female Zebra Finches show significant activation in the hippocampus and area AHP when exposed to conspecific songs compared to heterospecific ones, indicated by a higher density of Fos-immunoreactive cells (Bailey et al., 2002). This suggests that the hippocampus and AHP play a specific role in recognizing and responding to species-specific songs in females.
Sexual imprinting involves early learning where birds store information about their social environment to guide the selection of a suitable sexual partner. In Zebra Finches, the first courtship experience after reaching sexual maturity sharpens and consolidates their image of a sexual partner (Sadananda and Bischof, 2004). The hippocampus is believed to be involved in the consolidation of sexual imprinting, possibly facilitating the transfer of information to other telencephalic areas that undergo synaptic changes during this consolidation process. Additionally, in quails, the hippocampus is implicated in conditioned responses related to sexual behavior (Taziaux et al., 2007). These findings highlight the hippocampus' involvement in various behaviors critical to reproduction and partner selection in birds.
The regulation of the HPA axis, a central stress response system, involves the secreted glucocorticoid (GC) and their receptors—specifically, the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR) (Krause et al., 2016, 2017). Both types of receptors are expressed in the hippocampus of both birds and mammals (Hodgson et al., 2007; Dickens et al., 2009; Senft et al., 2016; Baugh et al., 2017). MRs have a higher affinity than GRs for CORT, the primary GC in birds and rodents, indicating that MRs are more active at baseline CORT levels, whereas GRs are more involved during acute peak levels (Lattin et al., 2022; Madison et al., 2024). Tree Swallows (Tachycineta bicolor) living in extreme environments exhibit higher MR expression in the hippocampus but not GR expression (Zimmer et al., 2023). Similarly, European Starlings (Sturnus vulgaris) under chronic stress show increased MR expression, with no change in GR expression (Dickens et al., 2009). Conversely, acute stress does not affect either MR or GR expression in the hippocampus of White-crowned Sparrows (Krause et al., 2021). These observations suggest that chronically high levels of baseline CORT can lead to increased hippocampal MR expression.
Studies have shown that CORT administration can enhance spatial abilities. For instance, in Mountain Chickadees, elevated CORT levels improve cache retrieval and spatial memory (Pravosudov, 2003). Chronic but moderate increases in CORT due to unpredictable food availability also correlate with improved spatial performance in these birds (Pravosudov and Clayton, 2002). However, experimentally elevated CORT levels during early development impair spatial abilities in Black-legged Kittiwakes (Rissa tridactyla; Kitaysky et al., 2003) and Zebra Finches (Hodgson et al., 2007). Chronic activation of the HPA axis by external stressors can negatively influence various aspects of hippocampal structure (Smulders, 2017; Kadhim et al., 2021). These findings suggest that while acute increases in CORT can enhance spatial abilities, chronic elevation impairs spatial learning and memory.
The avian hippocampus plays a role in regulating negative feedback of the HPA axis, similar to its role in mammals, as identified since the 1970s (Jacobson and Sapolsky, 1991). In pigeons, hippocampal inhibition of the HPA axis occurs through the suppression of hypothalamic activity, especially when the caudal hippocampus is stimulated, which reduces CORT levels (Bouille and Bayle, 1978). Additionally, hippocampal lesions can disrupt the circadian rhythm of CORT, maintaining high levels across all times.
Steroid hormones, including androgens and estrogens, play a vital role in spatial memory acquisition and retrieval by promoting hippocampal neurogenesis, synapse formation, and dendrite development (Luine, 2008; Barha and Galea, 2010; Mendez et al., 2011). The enzyme aromatase, which converts androgens to estrogens, is found in the hippocampus or analogous regions in many vertebrates, including birds (Hodgson et al., 2008; Azcoitia et al., 2011). This suggests a capacity for local estrogen production, supporting the conserved functional role of the hippocampus across vertebrate taxa (Vahaba and Remage-Healey, 2015).
Birds exhibit remarkable spatial memory capabilities and high hippocampal expression of aromatase compared to other brain regions (Fusani et al., 2001a, 2001b; Saldanha et al., 2004), suggesting that locally-produced estrogens may aid in spatial memory acquisition or retrieval (Bailey et al., 2013; Bailey and Saldanha, 2015). In Zebra Finches, aromatase inhibition impairs spatial memory acquisition and accuracy (Bailey et al., 2013, 2017), and antagonizing hippocampal estrogens with 1,4,6-adrostatriene-2,17-dione (ATD) worsens performance on spatial memory tasks, emphasizing the importance of aromatization for spatial memory (Bailey et al., 2013). These findings suggest that local estrogen activity is crucial for memory of cache locations, especially when circulating sex steroid hormone levels are low.
Hippocampal aromatase expression is notably high in various birds such as Zebra Finch (Saldanha et al., 2004; Bailey et al., 2013, 2017), Canary (Serinus canarius; Fusani et al., 2001b; Brown et al., 2023), and Ring Dove (Streptopelia risorii; Fusani et al., 2001a). Circulating testosterone (T) can be converted locally into estradiol (E2) to promote spatial memory acquisition in the songbird hippocampus. In Zebra Finches, implants of E2 or T stimulate spatial memory acquisition, enlarge hippocampal cell size, and improve memory retrieval (Oberlander et al., 2004). Similarly, oral E2 or T treatments in Great Tits (Parus major) show a trend toward improved memory recall (Hodgson et al., 2008). Using an aromatase inhibitor, fadrozole, combined with T in Zebra Finches increases error rates, underscoring the need for high E2 levels for spatial memory. Notably, these hormone effects are limited to the anterior hippocampus, aligning with seasonal changes in cell numbers and immediate early gene expression in the anterior hippocampus of some other songbirds, such as Black-capped Chickadee (Smulders and DeVoogd, 2000; Smulders et al., 2000).
The G protein-coupled estrogen receptor (GPER)-1, a membrane-bound estrogen receptor, is implicated in the rapid effects of E2 on memory function (Ervin et al., 2015; Gabor et al., 2015). In Zebra Finches, spatial memory task performance relies on local aromatase and E2 synthesis, potentially mediated by GPERs (Bailey et al., 2017). Postsynaptic density protein 95 (PSD95), found primarily in dendrites, interacts directly with glutamate receptors and other postsynaptic proteins to promote synaptic plasticity (Chen et al., 2011). In Zebra Finch hippocampal neurons, aromatase colocalizes with N-methyl-D-aspartic acid (NMDA)-type glutamate receptors, indicating that estrogens may influence synaptic plasticity through pre- and post-synaptic actions (Saldanha et al., 2004). E2 activation of these receptors can alter PSD95 or its targets, enhancing synaptic plasticity and memory (Bailey et al., 2017). These findings suggest that synaptic aromatization is vital for synaptic size and strength, and that E2 acts similarly to a neurotransmitter, integrating hormonal and electrical signaling at the synapse (Saldanha et al., 2011).
In summary, the endocrine sensitivity and connectivity of the avian hippocampus position it as a central neural hub. This region integrates sensory information with the internal states of birds, facilitating essential behaviors like spatial memory and navigation, which often involves three-dimensional space, as well as emotional, and endocrinological responses to cope with external environmental stimuli.
In summary, the avian hippocampus serves as an integrative neural hub that merges sensory inputs with internal physiological states, enabling birds to adaptively respond to their surrounding environments through spatial navigation, memory retrieval, as well as emotional regulation and stress response modulation. Its functions extend well beyond a mere cognitive map, encompassing emotional valence and motivational aspects within spatial contexts, and supporting a comprehensive behavior framework crucial for survival and reproductive success. The interactions between the hippocampus and the endocrine system highlight its role in managing stress physiology and reproductive behaviors such as song recognition and sexual imprinting. As local estradiol production in the hippocampus enhances memory and plasticity, this underscores the underlying neurophysiological mechanisms of the hormonal networks that facilitate spatial memory in birds. By integrating hormonal, emotional, behavioral, and environmental information, the avian hippocampus demonstrates multifaceted functions that are critical for environmental navigation, foraging, migration, and social interactions. Future research should focus on elucidating the precise mechanisms underlying these interactions that support extraordinary avian behaviors like food caching and navigation, enhancing our understanding of hippocampal function in vertebrates. This includes exploring the avian hippocampus' role in novel contexts and potential applications of these insights into understanding stress, memory, and cognitive functions in both avian and non-avian species.
Not applicable.
Juyong Li: Writing – review & editing, Writing – original draft. Jing-An Liu: Investigation. Limin Wang: Writing – review & editing, Writing – original draft, Investigation, Funding acquisition. Dongming Li: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Conceptualization.
The authors declare they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
| AMV | medial ventral arcopallium |
| APH | area parahippocampalis |
| ATD | 1,4,6-adrostatriene-2,17-dione |
| BDNF | brain-derived neurotrophic factor |
| CA | cornu ammonis |
| CORT | corticosterone |
| DCX | doublecortin |
| DG | dentate gyrus |
| DL | dorsolateral |
| DLd | dorso-dorsolateral hippocampus |
| DLv | ventro-dorsolateral hippocampus |
| DM | dorsomedial |
| DMd | dorso-dorsomedial hippocampus |
| DMv | ventro-dorsomedial hippocampus |
| E2 | estradiol |
| EC | entorhinal cortex |
| GC | glucocorticoid |
| GPER | G protein-coupled estrogen receptor |
| GPS | global positioning system |
| GR | glucocorticoid receptor |
| Hp | hippocampus proper |
| HPA | hypothalamic-pituitary-adrenal axis |
| IEGs | immediate early genes |
| MR | mineralocorticoid receptor |
| NCL | nidopallium caudale |
| NMDA | N-methyl-D-aspartic acid |
| PFC | prefrontal cortex |
| PSD95 | postsynaptic density protein 95 |
| PVN | paraventricular nucleus |
| T | testosterone |
| Tr | triangular hippocampus |
| V | ventral regions |
| Vl | ventrolateral hippocampus |
| Vm | ventromedial hippocampus |
| Function | Order | Family | Species | Treatment, stimulus, or measurement | Main points | References |
| Spatial memory | Passeriformes | Paridae | Black-capped Chickadee (Poecile atricapillus) | Food-caching, neuronal acitivity in hippocampus | An increase of the number of immediate early genes immunoreactive neurons within hippocampus after food hoarding. | Smulders and DeVoogd, 2000; Bailey et al., 2002; Ben-Tov and Gutfreund, 2022 |
| Hippocampal lesion | Disrupted spatial memory in food-storing birds. | Patel et al., 1997 | ||||
| Food-caching, hippocampal lesion | Disrupted birds' ability to recall the locations of stored or hidden seeds but do not impair the motivation to store. | Sherry et al., 1989 | ||||
| Food-caching, hippocampus volume | Food-caching species had bigger hippocampus relative to non-storing species. | Krebs et al., 1989; Healy and Krebs, 1992 | ||||
| Food-caching, neuronal density in hippocampus | Experience of food storing and retrieval resulted in hippocampus enlargement with an increase in neuronal number. | Clayton and Krebs, 1994 | ||||
| Tufted Titmouse (Baeolophus bicolor) | Food-caching | Accurate cache retrieval requires the hippocampus, food-caching birds are memory specialists, capable of remembering many scattered, concealed food locations. | Payne et al., 2021 | |||
| Icteridae | Brown-headed Cowbird (Molothrus ater) | Brood parasitism, hippocampus volume and hippocampal neurogenesis | Females, not males, remember locations of various potential host nests to monitor progress of nest-building and egg-laying. | Guigueno and Sherry, 2017 | ||
| Brood parasitism, hippocampus volume | Females performed better than males on a spatial task and had a larger hippocampus than males. | Sherry et al., 1993; Guigueno et al., 2014 | ||||
| Shiny Cowbird (Molothrus bonariensis) | Hippocampus volume | No sex differences were found in Screaming Cowbirds, a brood parasite in which males assist females in nest searching. | Reboreda et al., 1996 | |||
| Brood parasitism, hippocampus volume | Females had bigger hippocampus size than males, due to only females searching for nests. | Reboreda et al., 1996 | ||||
| Emberizidae | White-crowned Sparrow (Zonotrichia leucophrys) | Hippocampus volume and hippocampal neurogenesis | Migratory sub-species have a larger hippocampus and greater hippocampal neurogenesis than non-migratory sub-species. | Pravosudov et al., 2006; LaDage et al., 2011 | ||
| Sylviidae | Reed Warbler (Acrocephalus scirpaceus) | Hippocampal neurogenesis | Hippocampal neurogenesis is greater in a migratory Reed Warbler than their closely related non-migratory species. | Barkan et al., 2014 | ||
| Hirundinidae | Purple Martin (Progne subis) | Hippocampus volume and hippocampal neurogenesis | Hippocampus size and neurogenesis are closely associated with patterns of space use. | Lalla et al., 2022 | ||
| Estrildidae | Zebra Finch (Taeniopygia guttata) | Acquisition and retention of spatial learning | The hippocampus is involved in the storage as well as in the retrieval of spatial memory. | Watanabe and Bischof, 2002, 2004; Bischof et al., 2006; Mayer et al., 2010 | ||
| Acquisition and retention of spatial learning | Expression of immediate early genes (c-fos and ZENK) is up-regulated in hippocampus during spatial task learning and recall. | Bischof et al., 2006; Mayer et al., 2010 | ||||
| Hippocampal lesion, acquisition and retention of spatial learning | Affected the acquisition and retention of spatial learning tasks and lead to deficits in spatial memory. | Watanabe and Bischof, 2002, Watanabe and Bischof, 2004; Bischof et al., 2006 | ||||
| Acute stress | High CORT response induced by acute stress impaired spatial learning and memory. | Hodgson et al., 2007 | ||||
| Inhibiting hippocampal estrogens | Inhibiting hippocampal estrogens by antagonizing aromatase with ATD (1,4,6-adrostatriene-2,17-dione), caused worse spatial memory. | Bailey et al., 2013 | ||||
| Charadriiformes | Scoiopacidae | Semipalmated Sandpiper (Calidris pusilla) | Hippocampus volume | Hippocampus volume was larger in wintering birds than migrating birds. | de Morais Magalhaes et al., 2017 | |
| Spatial navigation | Columbiformes | Columbidae | Homing Pigeon (Columba livia) | Hippocampus volume | Those individuals with developmental experience navigating to a home loft had larger hippocampi than those that did not. | Cnotka et al., 2008 |
| Hippocampal lesion | Caused a profound loss of navigational abilities. | Colombo et al., 2001 | ||||
| Hippocampal lesion | Lost their abilities to orient their vanishing bearings towards home from a familiar training site. Impaired performance before learning, but not when maps were already acquired. | Gagliardo et al., 2009; Gagliardo, 2013; Coppola et al., 2014b | ||||
| Hippocampal activity | Upregulated hippocampal neuronal activation when they homed from a familiar location. | Shimizu et al., 2004 | ||||
| Hippocampal lesion | Impaired spatial learning behavior in a radial maze task, and performed poorly on spatial autodiscrimination task. | Watanabe, 1999; Colombo et al., 2001 | ||||
| Galliformes | Phasianidae | Domestic Chicken (Gallus gallus) | Spatial encoding of goal location | The hippocampus is instrumental for enabling the spatial encoding of goal locations. | Tommasi et al., 2003; Morandi-Raikova and Mayer, 2021 | |
| Visual-spatial perception | Columbiformes | Columbidae | Homing Pigeon (Columba livia) | Hippocampal lesion | Take a straighter path home compared to controls. | Gagliardo et al., 2014; Herold et al., 2015 |
| Hippocampal lesion | More likely to display high frequency, oscillatory shifts in their flight paths. | Gagliardo et al., 2023 |
| Function | Order | Family | Species | Treatment, stimulus, or measurement | Main points | References |
| Anxiety-related behavior | Columbiformes | Columbidae | Homing Pigeon (Columba livia) | Hippocampal lesion, approach-avoidance conflict | Ignore the presence of a human in the testing room. | Broadbent and Colombo, 2000 |
| Galliformes | Phasianidae | Domestic Chicken (Gallus gallus) | Long tonic immobility | Laying hens had higher expression of proliferating cell nuclear antigen in hippocampus. | Armstrong et al., 2020b | |
| Approach-avoidance conflict, passive avoidance learning test | Those learned to avoid a bitter event (the methyl anthranilate bead) showed reduced number of newly-generated cells in hippocampus. | Nikolakopoulou et al., 2006 | ||||
| Japanese Quail (Coturnix japonica) | Social isolation stress | Exhibited c-Fos reactivity especially in the dorsolateral area of hippocampus. | Takeuchi et al., 1996 | |||
| Long tonic immobility | Fewer newly-generated cells in hippocampus. | Lormant et al., 2020 | ||||
| Passeriformes | Passeridae | House Sparrow (Passer domesticus) | Approach-avoidance conflict, neophobic behavior | Increased with those gene expressions of caudal hippocampus, i.e., increased neuronal activity in response to novel objects. | Lattin et al., 2022; Kimball et al., 2022 | |
| Stress-related physiology | Passeriformes | Paridae | Black-capped Chickadee (Poecile atricapillus) | Captivity | Reduced hippocampal volume. | Tarr et al., 2009 |
| Captivity | Reduced hippocampal neurogenesis. | Barnea and Nottebohm, 1994 | ||||
| Mountain Chickadee (Poecile gambeli) | Social ranking | Subordinate individuals had lower levels of cell proliferation in the hippocampus than dominant birds. | Pravosudov and Omanska, 2005 | |||
| Captivity | Captive individuals have less newly-generated neurons relative to those wild individuals. | LaDage et al., 2010 | ||||
| CORT administration | Showed superior cache retrieval and spatial memory performance compared with control birds. | Saldanha et al., 2000; Pravosudov, 2003 | ||||
| Unpredictable food availability | Small but chronic elevations in CORT triggered correlated with enhanced cache retrieval and performance. | Pravosudov and Clayton, 2001 | ||||
| Icteridae | Brown-headed Cowbird (Molothrus ater) | Captivity | Reduced hippocampal volume. | Day et al., 2008 | ||
| Emberizidae | White-crowned Sparrow (Zonotrichia leucophrys) | Acute stress | Nt changes either MR or GR expression in the hippocampus. | Krause et al., 2021 | ||
| Estrildidae | Zebra Finch (Taeniopygia guttata) | Dim light at night | Increased proliferation in the ventricular zone adjacent to hippocampus, and increased recruitment and total neuron numbers in hippocampus. | Moaraf et al., 2020a, Moaraf et al., 2020b, Moaraf et al., 2021 | ||
| Domestic Chicken (Gallus gallus) | Chronic food restriction | Reduced the number of newly-generated neurons in hippocampus. | Robertson et al., 2017 | |||
| Corvidae | Indian House Crow (Corvus splendens) | Constant light | Decreased DCX + neurons, smaller neuronal somata, fewer glia in hippocampus. | Taufique et al., 2018a, Taufique et al., 2019 | ||
| Dim light at night | Decreased the expression of several hippocampal genes including BDNF. | Taufique et al., 2018b | ||||
| Passeridae | House Sparrow (Passer domesticus) | Captivity | Declined dendrite length and spine density of hippocampal neurons. | Roth 2nd et al., 2017 | ||
| Emberizidae | Dark-eyed Junco (Junco hyemalis) | Captivity | Reduced hippocampal volume. | Smulders et al., 2000 | ||
| Sylviidae | Garden Warblers (Sylvia borin) | Captivity | No difference in hippocampus volume in response to captivity for 5 days or 1 year. | Healy et al., 1996 | ||
| Hirundinidae | Tree Swallow (Tachycineta bicolor) | Extreme environments | Increased MR mRNA expression but not GR in hippocampus. | Zimmer et al., 2023 | ||
| Sturnidae | European Starlings (Sturnus vulgaris) | Chronic stress | Increased MR mRNA expression in hippocampus. | Dickens et al., 2009 | ||
| Galliformes | Phasianidae | Domestic Chicken (Gallus gallus) | Acute (24 h) food deprivation | Reduced spine numbers in hippocampus. | Kumar et al., 2023 | |
| Lariformes | Laridae | Blacklegged Kittiwake (Rissa tridactyla) | CORT administration | Compromised spatial ability during early development. | Kitaysky et al. 2003 | |
| Sexual behavior | Galliformes | Phasianidae | Japanese Quail (Coturnix japonica) | Conditional stimulus of sexual behavior | Exhibited FOS-immunoreactive fibers in hippocampus. | Taziaux et al., 2007 |
| Song learning | Passeriformes | Estrildidae | Zebra Finch (Taeniopygia guttata) | Conspecific or heterospecific song stimuli | Higher density of FOS-immunoreactive cells in hippocampus when they heard conspecific learned song. | Bailey et al., 2002 |

