| Citation: | Jiaojiao Wang, Peng Pan, Haijie Zhang, Laikun Ma, Qindong Zhou, Longwu Wang, Jianhua Hou. 2025: Nestlings of two parrotbill species can independently evaluate the presence of predators based on acoustic cues. Avian Research, 16(1): 100217. DOI: 10.1016/j.avrs.2024.100217 |
Nest predation is the leading cause of reproductive failure in birds and a major driving force in the evolution of anti-predation strategies. Current studies on the anti-predation strategies of birds driven by predation pressure have mainly focused on adults. However, the detection and behavioral responses of nestlings toward predation risk require further investigation. In this study, we examined nestling responses to predator sounds. Two species of nestlings, the Reed Parrotbill (Paradoxornis heudei) and Vinous-throated Parrotbill (Sinosuthora webbiana), were exposed to the Sparrowhawk (Accipiter nisus, less common) and Oriental Magpie (Pica serica, more common), which are predator species, the Oriental Turtle Dove (Streptopelia orientalis), which is a commonly found harmless species, and background noise. Our findings revealed that compared to pre-playback of natural begging and playback of background noise and Oriental Turtle Dove sounds, playback of the two predator types decreased the total begging time and total number of calls of the two nestlings species, with the calls of the Sparrowhawk leading to greater suppression of nestling begging behavior than those of the Oriental Magpie. Therefore, our results indicated that these nestlings were able to distinguish predators from harmless species based on auditory cues.
In natural communities, nearly all animals experience threats from multiple predators (Sih et al., 1998). In response to such predation pressure, many animals have evolved various anti-predation strategies to enhance their fitness (Ghalambor and Martin, 2001; Hua et al., 2014; Schneider and Griesser, 2014). Effective anti-predation behavior is predicated upon the ability to recognize predators with different threat levels, which can significantly enhance the adaptability and survival rate of individuals under attack (Caro, 2005). Studies have shown that numerous animals, particularly birds, are able to identify predators through various cues, including sight (Gill et al., 1997; Trnka et al., 2015), sound (Rainey et al., 2004; Yu et al., 2016), and smell (Amo et al., 2008). Birds use vision to quickly identify predator features (Shang, 2018), such as raptors' hooked beaks (Gill et al., 1997). Moreover, their hearing system allows them to sensitively recognize the sounds of predators and alarm signals of their companions; for example, Barn Swallows (Hirundo rustica) can recognize the alarm calls of specific predators (Yu et al., 2016). Smell also plays a role in risk recognition. For example, Blue Tits (Cyanistes caeruleus) are capable of sensing weasel odors, thus reducing the time spent in the nest (Amo et al., 2008). These abilities significantly enhance the birds’ adaptability and chances of survival.
Different mechanisms have been proposed to explain how animals acquire the ability to recognize predators. First, animals may possess a genetically programmed capacity for predator recognition that enables them to recognize predators in the absence of previous contact or endows them with extremely high sensitivity to specific stimuli, thus causing predator recognition to trigger innate responses (Dessborn et al., 2012; Veen et al., 2000). Second, predator discrimination can be learned by observing how conspecific (Griffin, 2009) or heterospecifics animals (Fallow et al., 2013) react to potential predators or through direct interaction with predators (Chivers and Ferrari, 2013). For example, Berger et al. (2001) found that auditory predator recognition in Moose (Alces alces) is learned, with naive Moose being capable of processing information about new predators within a generation.
Altricial nestlings mainly solicit food from their parents by exhibiting begging behaviors. Although begging can convey a higher demand for food, it can also incur certain costs, including increased energy consumption (Wright and Leonard, 2002) and chance of exposure, leading to increased risks of detection and predation by predators (Haskell, 1994; Briskie et al., 1999; Haff and Magrath, 2011). In contrast with adults, nestlings are considerably more vulnerable when faced with danger because they lack effective means of escape or defense. Therefore, altricial nestlings depend predominantly on their parents for external risk information, and nestlings lower their chances of detection by decreasing their begging and activity (Ibáñez-Álamo et al., 2015; Wang et al., 2022). Nestlings can acquire risk information through parental alarm calls (Wang et al., 2022). They are even able to identify the type of alarm call and adjust their behavior accordingly. For example, when the nestlings of Japanese Tits (Parus minor) heard parental alarm calls in response to Jungle Crows (Corvus macrorhynchos), they crouched down inside the nest and suppressed begging (Suzuki, 2011). In contrast, when the nestlings heard parental alarm calls in response to Japanese Rat Snakes (Elaphe climacophora), they fled the nest (Suzuki, 2011). Nestlings of Noisy Miners (Manorina melanocephala) suppressed their begging behavior over a longer period in response to conspecific terrestrial alarm calls than in response to aerial alarm calls (Barati and McDonald, 2017). However, as parents cannot always be present around the nest, it is crucial for the nestlings to independently assess the presence of predators.
Adults can accurately identify intruders using specific cues, e.g., acoustic signals (Rainey et al., 2004; Yu et al., 2016; Lawson et al., 2020; Wang et al., 2024a). However, nestling awareness of cues that signal danger may be more subtle than previously reported. A number of studies have found that nestlings can perceive external risks through visual cues, thus displaying avoidance behaviors at the approach of the observers or other stimuli. However, this may mainly be the case for precocial nestlings or older altricial nestlings (Schaller and Emlen, 1961). At the very least, this behavior implies that older nestlings can assess risks independent of their parents. Previous experiments on two-day-old nestlings of the Australian Brush Turkey (Alectura lathami) using different predator specimens showed that the nestlings displayed crouching or running behavior depending on the predator type (Göth, 2001). Domestic poultry chicks (Gallus gallus) began avoiding cat odors at seven days old (Fluck et al., 1996). Compared to visual and olfactory cues, acoustic signals offer several advantages, such as ability to operate in the dark, not being hindered by objects, and ability to travel over long distances (Slabbekoorn and Smith, 2002; Whittingham et al., 2004). Therefore, acoustic cues may be more useful for species in closed nests or environments with limited visibility. Furthermore, nestlings can perceive these cues before their vision is adequately developed (Khayutin, 1985). Limited research has suggested that nestlings respond to predator sounds by suppressing their begging (Leonard et al., 2005; Magrath et al., 2007; Haff and Magrath, 2010; Fuchs et al., 2019). For example, nestlings of the White-browed Scrubwren (Sericornis frontalis) suppress begging in response to acoustic cues from a predator walking on leaf litter (Magrath et al., 2007); however, the nestlings responded less to synthetic broadband sounds and did not respond to novel sound (Haff and Magrath, 2010). Leonard et al. (2005) found that nestlings exposed to replays of predator (Common Grackle Quiscalis quiscala) landing on the nest box showed a reduced rate and intensity of begging compared to those exposed to replays of adult Tree Swallows (Tachycineta bicolor) landing on the nest box. Therefore, further investigations are needed to verify whether nestlings can distinguish among different types of predators based on sound.
In this study, we examined the nestlings of two sympatric species in the Baiyangdian Lake region of Hebei, China: the Reed Parrotbill (Paradoxornis heudei) and Vinous-throated Parrotbill (Sinosuthora webbiana). Recorded sounds from two types of predators were played back to the nestlings, namely those of the Sparrowhawk (Accipiter nisus), which preys on adult birds, eggs, and nestlings but is rarely observed during breeding season, and the Oriental Magpie (Pica serica), which mainly preys on eggs and nestlings and is commonly found in the area. We also played sounds from a harmless species, the Oriental Turtle Dove (Streptopelia orientalis), along with background noise. The aim of these experiments was to explore whether the two species of nestlings were able to distinguish between the sounds of predators and harmless species and between the sounds of different predators.
The study site is located at Baiyangdian Lake, Hebei Province, China (38°43′–39°10′ N, 115°38′–116°19′ E, 10 m a.s.l.), which is in a warm, temperate, and semi-arid continental monsoon climate zone. The annual average air temperature of the study site is 12.2 ℃, extreme maximum temperature is 40.7 ℃, and extreme minimum temperature is −26.7 ℃. The annual average precipitation and evaporation are 529.7 and 993.0 mm, respectively (He et al., 2022).
The two sympatric breeding birds selected for this study, the Vinous-throated Parrotbill and Reed Parrotbill both belong to the family Paradoxornithidae and order Passeriformes. Both species breed in reedbeds. Local predators include snakes, Siberian Weasels (Mustela sibirica), Oriental Magpies, and raptors. The Oriental Turtle Dove is harmless to the Vinous-throated Parrotbill and Reed Parrotbill.
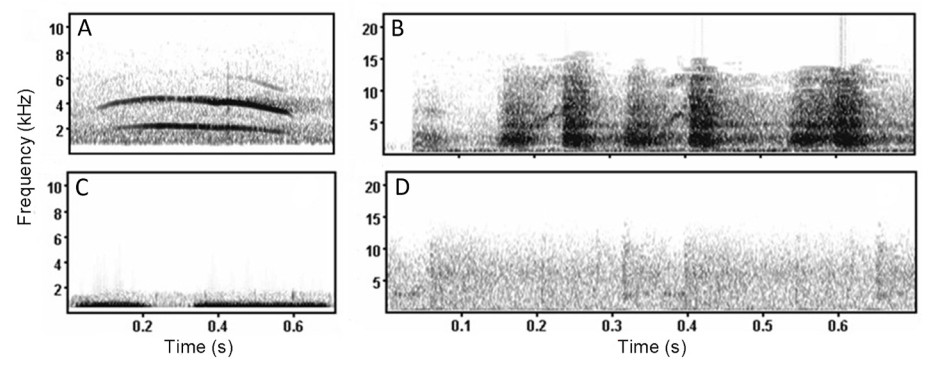
The calls of Oriental Magpie (XC401642 and XC401643: Beijing, China, 2018; XC750058: Beijing, China, 2022), Sparrowhawk (XC309239: Andalucía, Spain, 2016; XC87380: Madeira, Portugal, 2011), and Oriental Turtle Dove (XC723155 and XC761097: Beijing, China, 2022; 2021 respectively) were downloaded from XenoCanto recordists (http://www.xeno-canto.org/). Raven Pro 1.4 (version 1.4; Cornell Laboratory of Ornithology, Ithaca, NY) was used to select the clear sound clips and delete parts that overlapped with other bird sounds (Fig. 1). Two calls were cropped for each species to minimize pseudoreplication. The duration of each sound type was 30 s. Meanwhile, the background noises of two samples were extracted from the sounds of three bird species and clipped to 30 s. All sounds were stored as WAV audio files.
From May to July 2023, we conducted playback experiments on nestlings of the two species (nestlings of Vinous-throated Parrotbill: n = 15; nestlings of Reed Parrotbill: n = 13). We used the experimental methods detailed in previous studies (Madden et al., 2005a, 2005b; Bernath-Plaisted and Yasukawa, 2011) to conduct a playback experiment on nestlings at approximately seven days old. Nestlings of this age are able to strongly beg in response to human stimulation. In addition, the nestlings at this age are relatively less fragile and thus can be easily transported from the wild to the indoor playback experiment. Because the playback of predator sounds in the wild may induce adult birds to create alarm calls and thus affect the response of the nestlings, we conducted the experiment in an indoor location near the study site. One nestling was randomly selected from each breeding nest for playback.
A nestling was placed in the old nest that we collected, starved for 40 min, and got habituated to the environment. Manually touching the edge of the nest prompted the nestling to make begging calls. The nest was touched every 3 s within a duration of 30 s, and the begging behavior was recorded to compare with the begging behavior following playback. A digital video recorder placed 0.5 m away from the nest was used to record the chicks' begging behavior. After that, the nestling was calmed down for 1 min before we began to play the sounds through a Bluetooth speaker (QoyQueen T350, Shenzhen, China) 0.5 m away from the nest. One of the four types of sounds was selected at random (i.e., sparrowhawk calls, dove calls, magpie calls, or background noise), and a sample was randomly selected from each sound for playback. The playback time was 30 s. The order of playback of the four sounds was random, and the interval between sounds was 5 min. The volume of all sounds was ~70 dB at 1 m away from the speaker. Each nestling was subjected to all four types of sounds. As with the operation before the sound was played, the nestling was encouraged to beg by manually touching the nest to simulate the parent bird’s return to the nest. The same researcher (P.P.) completed all experimental procedures. When the experiment was complete, the nestlings were quickly returned to their original nest.
Based on the recorded video, we quantified the two parameters representing the begging behavior of the nestlings, i.e., total number of calls and total begging time. The nestling begging behavior was quantified indoors by Q.Z.
We measured the following sound parameters to identified the fundamental soundwaves of each of the three species (dove, sparrowhawk and magpie: five samples): low frequency, high frequency, bandwidth, peak frequency, syllable duration, and syllable rate (Appendix Table S1).
Generalized linear mixed models (glmmTMB in R 4.1.3) with Poisson distribution were employed to analyze the recognition of sympatric bird nestlings for different predator sounds. The total number of calls and total begging time were defined as the target variables. Playback type (natural begging, background noise, dove calls, magpie calls, and sparrowhawk calls) was included as a fixed effect. The body weight of the nestlings was included as a covariate, and the nestling identity was defined as a random effect. We used two-tailed likelihood ratio tests to obtain P values. For significant between-group results, post hoc comparisons were performed between the two groups, and the P values of the between-group comparisons were corrected using Holm’s adjustment. In addition, to avoid the effect of presentation order on the begging behaviors of the nestling, non-parametric tests (total begging time of Vinous-throated Parrotbill and Reed Parrotbill; total number of calls of Vinous-throated Parrotbill) or ANOVA (total number of calls of Reed Parrotbill) were also performed to verify the differences in begging behaviors among different presentation orders. Finally, we also compared the sound parameters of the three species used in the playback in an attempt to identify the parameters that affected the response of the nestlings. One-way ANOVA was used for sound parameter data that conformed to normal distribution, while non-parametric tests were used for sound parameter data that conformed to a non-normal distribution. The data of the effect of the presentation order on the begging behavior of nestlings and data of sound parameter were performed using IBM SPSS 26.0 for Windows (International Business Machines Corporation, New York, USA). All tests were two-tailed, and the significance level was 0.05.
The generalized linear mixed models indicated that playback type had significant effects on the total begging time (Vinous-throated Parrotbill: χ2 = 309.347, P < 0.001; Reed Parrotbill: χ2 = 208.851, P < 0.001) and total number of calls (Vinous-throated Parrotbill: χ2 = 436.360, P < 0.001; Reed Parrotbill: χ2 = 311.647, P < 0.001). Multiple comparison tests revealed that for the two species, the magpie and sparrowhawk sounds significantly decreased their total begging time and total number of calls compared to natural begging, background noise, and dove calls (P < 0.05; Appendix Table S2). In addition, the sparrowhawk calls led to significantly lower begging behaviors of nestlings than the magpie calls (Figs. 2 and 3; Appendix Table S2). Furthermore, significant differences were not observed in the total begging time of the two nestling species among the pre-playback natural begging, background noise, and dove calls (P > 0.05) (Fig. 2; Appendix Table S2). However, for both species, playback of background noise and doves calls significantly reduced the total number of calls by nestlings compared to the number of natural begging calls (P < 0.01) (Fig. 3; Appendix Table S2). Compared to number of calls in response to dove calls, the number produced by the Vinous-throated Parrotbill nestlings was significantly higher when background noise was played back (z = 2.180, P = 0.029); however, the difference between the two was not significant in the Reed Parrotbill nestlings (z = −0.091, P = 0.928) (Fig. 3; Appendix Table S2).
We found that the body weight of the nestlings had no effect on the two response variables of Vinous-throated and Reed Parrotbills (P > 0.05). In addition, we found that the playback order had a certain effect on the begging behavior of the two species, but the difference was only significant between the first order and other orders (P < 0.05). There was no significant difference in begging behavior among the other orders (P > 0.05).
On the analysis of sound parameters, the results showed that these parameters significantly differed among the three species (P < 0.05). The multiple comparison results showed that there were significant differences in each sound parameter except for the following three groups: low frequency between sparrowhawk and magpie (P > 0.05), the high frequency between sparrowhawk and dove (P > 0.05), and the high frequency between sparrowhawk and magpie (P > 0.05).
Our findings indicated that the playback of sparrowhawk and magpie calls caused the nestlings to significantly suppress their begging behavior compared to pre-playback of natural begging and playback of background noise and dove calls. This finding suggests that the two species of nestlings were able to distinguish between the sounds of predators and other harmless species and effectively adjust their begging behavior upon hearing these calls to reduce their predation risk, thereby enhancing their individual fitness.
When nestlings solicit food from their parents through begging, they may also attract the attention of predators (Haff and Magrath, 2011). Hence, predation pressure has driven the evolution of corresponding anti-predation strategies in birds. For example, Blackcaps (Sylvia atricapilla) have a high predation rate, and the nestlings of this species only develop begging calls just before they are able to leave the nest (Węgrzyn and Leniowski, 2015). Nestlings are also able to recognize predator-related information in parental alarm calls and respond appropriately, thereby enhancing their individual fitness (Suzuki, 2011; Wang et al., 2022). This finding is similar to the results of previous research on parrotbill nestlings, which were found to eavesdrop distress calls from their sympatric prey nestlings and then reduce their begging behavior (Wang et al., 2024b). In the present study, Vinous-throated and Reed Parrotbill nestlings displayed reduced begging behaviors after hearing predator sounds, which is also a type of anti-predation strategy. Similar to our findings, studies on the nestlings of Red-winged Blackbirds (Agelaius phoeniceus) (Yasukawa et al., 2020) and White-browed Scrubwrens (Magrath et al., 2007; Haff and Magrath, 2010) showed that these nestlings also suppressed begging or fell silent in response to predator sounds. Leonard et al. (2005) found that compared to the sound of a Tree Swallow adult landing on a nest box, that of the nest predator, the Common Grackle decreased the begging rate and begging intensity of Tree Swallow nestlings.
The threat posed by a given predator may vary greatly among different animals (Fuchs et al., 2019). Hence, the ability to recognize and respond appropriately to predators with different threat levels is a crucial skill that can improve prey fitness (Caro, 2005). For example, adult Black-capped Chickadees (Poecile atricapillus) can discriminate between the calls of high- and low-threat predators (Congdon et al., 2020). In the present study, although the Vinous-throated and Reed Parrotbill nestlings showed reduced begging behaviors in response to sparrowhawk calls relative to magpie calls, strong evidence of predator identification was not observed. The nestlings showed stronger response to sparrowhawk calls than to magpie calls, which may be explained by several factors. First, while predators are usually quiet when hunting, adult individual prey can spot predators when they are not hunting because predators usually live and socialize near them. Recent studies have found that many animals do respond to predator sounds or predator-related sounds (Blumstein et al., 2008). The long-term coexistence and evolution between adults and predators may have allowed parents to pass to their nestlings on the ability to recognize and avoid sparrowhawks. Second, sparrowhawks were less common than magpies in the study area; therefore, the nestlings took sparrowhawk calls as an unfamiliar sound and responded more strongly. Third, birds of prey have specific acoustic characteristics, such as pitch and frequency. Animals are more sensitive to this characteristic sound. Previous studies have found that Yellow-bellied Marmots (Marmota flaviventris) show stronger responses to eagles’ normal calls than they do to their reverse calls, suggesting that marmots associate normal calls with predators and do not just rather than just respond to fast-paced, wide-ranging calls. Specifically, the fact that completely novel reverse calls did not elicit an increase in alert response suggests that novelty is not the only factor influencing response (Blumstein et al., 2008). In addition, compared with the number of calls observed for natural begging, the number was significantly lower when the background noise and dove calls were played. This result may be related to the fact that the nestlings grew hungrier as the duration of food deprivation lengthened; therefore, the number of calls was reduced to reduce energy consumption (Chappell and Bachman, 2002). Visual signals involve nestlings opening their mouths to beg, whereas auditory signals involve nestlings making calls, which undoubtedly consumes more energy. Alternatively, natural (no playback) begging behaviors of nestlings were always tested first, and when the nestlings were “cheated” once (in the test without playback), the number of calls elicited in latter stimuli may have decreased. In addition, we found that playback order influenced the begging behavior of the nestlings because natural begging was always recorded first. In other words, when hearing a certain sound, the nestlings did not respond differently based on the order of playback.
The responses of birds to predators may be related to innate mechanisms (Veen et al., 2000; Dessborn et al., 2012) and learning (Maloney and Mclean, 1995; McLean et al., 1999; Kullberg and Lind, 2002). Our study on the responses of seven-day-old nestlings to the predators revealed that the nestlings reduced their begging behavior upon hearing the calls of the two predators. This behavior may be an innate response triggered by the pressure from multiple predators in the study site. According to data from 2022 to 2023, an average of approximately 140 Vinous-throated Parrotbill and 50 Reed Parrotbill nests were found each year, and the probability of nest predation for the two species was estimated to be 37% (personal observation). Sparrowhawk is not common in the breeding season in this area, and nestlings do not have many opportunities to associate sparrowhawk calls with predation events; therefore, the nestlings' response to sparrowhawk sounds may be instinctive. However, evidence suggesting that nestlings of both species learn magpie sounds from watching adults or from their own interactions with magpies is insufficient. In addition, whether nestlings respond to predator sounds in the absence of predator pressure, adult birds, and companions is unclear; therefore, further research is needed on nestlings' recognition mechanisms. Some acoustic parameters may also influence the nestlings’ responses, even if they cannot recognize different predators. These dynamics need to be validated in the future by artificially synthesizing or modulating predator sounds. Other studies have demonstrated that learning is also a mechanism for predator recognition. For example, a study on 8–15-month-old predator-naive Burrowing Bettongs (Bettongia lesueur) reported that the ability to recognize novel predators can be induced through experience (Steindler et al., 2020). However, Blue Tits and Great Tits (Parus major) did not respond to the novel predators (Carlson et al., 2017).
In the present study, the nestlings suppressed their begging behavior in response to predator sounds. This finding suggests that nestlings can use auditory signals to distinguish predators from non-threatening sounds. However, whether the nestlings of the two species can also visually recognize predators has not been studied.
In this study, we found that the nestlings were able to distinguish between the sounds of predators and harmless species. When nestlings hear a predator, they quickly engage in counter-predatory behavior that reduces begging. This rapid response may be key to their survival when faced with threats from natural predators. However, whether the nestling’s response to predator sounds is related to an innate response induced by high predation stress requires further study.
Jiaojiao Wang: Writing – original draft, Funding acquisition, Formal analysis, Conceptualization. Peng Pan: Investigation, Formal analysis. Haijie Zhang: Formal analysis, Data curation. Laikun Ma: Writing – original draft, Funding acquisition. Qindong Zhou: Data curation. Longwu Wang: Writing – review & editing, Funding acquisition. Jianhua Hou: Writing – review & editing.
The experiments reported here comply with the current laws of China. Fieldwork was carried out under permission from Yongnianwa National Wetland Park. Experimental procedures were in agreement with the Animal Experiment Ethics Committee of Guizhou Normal University (No. 2022001).
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank the Baiyangdian Nature Reserve for its support and permission to carry out this study. We also thank the anonymous reviewers whose feedback improved the quality of our manuscript.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2024.100217.
|
Caro, T.M., 2005. Antipredator Defenses in Birds and Mammals. University of Chicago Press, Chicago, IL.
|
|
Chappell, M.A., Bachman, G.C., 2002. Energetic costs of begging behaviour. In: Wright, J., Leonard, M.L. (Eds.), The Evolution of Begging: Competition, Cooperation and Communication. Kluwer Academic, Dordrecht, pp. 143–162.
|
|
Fuchs, R., Veselý, P., Nácarová, J., 2019. Predator Recognition in Birds: the Use of Key Features. Springer, Cham.
|
|
Khayutin, S.N., 1985. Sensory factors in the behavioral ontogeny of altricial birds. Adv. Stud. Behav. 15, 105–152.
|
|
Schneider, N.A., Griesser, M., 2014. Within-season increase in parental investment in a long-lived bird species: investment shifts to maximize successful reproduction? J. Evol. Biol. 28, 512–520.
|
|
Shang, Y., 2018. Behavioral Ecology, second ed. Peking University Press, Beijing.
|
|
Wright, J., Leonard, M.L., 2002. The Evolution of Begging: Competition, Cooperation and Communication. Springer, Dordrecht.
|