| Citation: | Wenzhi Yang, Yue Shen, Yuquan Miao, Zhaocun Lin, Yingmei Zhang. 2024: Genetic benefits of female from extra-pair paternity are context dependent within the socially monogamous Tree Sparrow. Avian Research, 15(1): 100203. DOI: 10.1016/j.avrs.2024.100203 |
Females actively seek extra-pair paternity (EPP) to acquire a fitness advantage for their offspring. The “context-dependence hypothesis” posits that female extra-pair mate choice has plasticity in response to environmental conditions, and therefore magnitude of female genetic benefits from EPP depends on the environmental variation. Furthermore, chronic heavy metal pollution can cause adverse effects on fitness-related traits of wild birds. However, few studies were available on the interactions between heavy metal pollution and EPP. We selected an area that was contaminated by heavy metals for more than 60 years (Baiyin, BY), a relatively unpolluted area (Liujiaxia, LJX), and Tree Sparrows (Passer montanus) as study species to explore the response of female extra-pair mate choice and genetic benefits from EPP to heavy metal pollution in socially monogamous birds. The relatedness between social mates and extra-pair mates was investigated. Additionally, we compared the body size and heterozygosity of extra-pair offspring (EPO), within-pair offspring (WPO), social males and extra-pair males from the two Tree Sparrow populations. We found that at BY, female Tree Sparrows tended to choose extra-pair males with larger body size and lower genetic similarity, thereby producing higher heterozygosity and larger body size of EPO compared with those of WPO. However, no similar phenomenon was observed in the Tree Sparrow population from LJX. In addition, there was a significant interaction between population and paternity in the analyses of the fitness-related traits, suggesting that environmental variation could affect female genetic benefits from EPP. This study confirmed the existence of presumed interactions between environmental pollution and EPP within the natural population of socially monogamous Tree Sparrows. Our findings shed lights on the possible effects of long-term environmental stress on mating system in wild birds.
Extra-pair paternity (EPP) is a common behavioral reproductive strategy by which male can benefit from gaining additional offspring in socially monogamous birds (Petrie and Kempenaers, 1998; Santema and Kempenaers, 2023). Interestingly, females of many species appear to freely engage or actively solicit EPP (Kempenaers et al., 1992; Jennions and Petrie, 2000; Brouwer and Griffith, 2019). Over the past decades, genetic benefit hypotheses have been proposed to account for the EPP (Brouwer and Griffith, 2019; Dobson et al., 2023). Specifically, females could select genetically superior (good gene hypothesis) or complementary (genetic compatibility hypothesis) males as extra-pair mates to ensure that offspring inherit specific genes (Johnsen et al., 2000; Foerster et al., 2003). The two hypotheses, while independent, are not mutually exclusive. In either case, extra-pair offspring (EPO) should on average have higher genetic qualities compared with their maternal half-siblings (within-pair offspring, WPO). Collectively, many studies have shown that EPO are superior to WPO in a number of fitness-related traits, including morphological parameters (Garvin et al., 2006), body condition (Sheldon et al., 1997; Charmantier et al., 2004; Freeman-Gallant et al., 2006), fledging rate (Whittingham and Dunn, 2001; Foerster et al., 2003; Charmantier et al., 2004), immune function (Johnsen et al., 2000; Garvin et al., 2006; Fossoy et al., 2008), heterozygosity (Foerster et al., 2003; Stapleton et al., 2007; Fossoy et al., 2008) and fecundity (Hasselquist et al., 1996; Schmoll et al., 2005). The above studies suggested that female birds can acquire good or compatible genes for their offspring from EPP. Furthermore, the “sex-allocation theory” predicts that females tend to produce offspring of sex with greater fitness potential (Johnson et al., 2009). Reproductive success rate in males is usually greater than that in females in socially monogamous birds with EPP (Whittingham and Dunn, 2005). Accordingly, successful males have the potential to produce more offspring and provide greater fitness returns for parents than successful females (Delmore et al., 2008). Thus, EPP will increase the variance in fitness of sons compared with daughters. In situations where EPO have greater fitness potential than WPO, females could bias the sex of EPO towards male. However, some other studies failed to reveal any differences between EPO and WPO (Barber et al., 2005; Forsman et al., 2008; Lee, 2012), even in sibling species or the same species. The seemingly contradictory results can be explained by the “context-dependence hypothesis” (Schmoll, 2011). Specifically, fitness-related traits are often affected by environment-specific gene expression (Arct et al., 2013). Female could select extra-pair mate with specific fitness-related traits under different environmental condition when genotype-by-environment interactions are present. Subsequently, female genetic benefits from EPP could also be changed by environmental factors.
It is reported that increased occurrence of EPP was associated with environmental variation (Pipoly et al., 2019). Furthermore, female genetic benefits from EPP could become more visible under specific environments (Arct et al., 2013), which have been formally tested in some passerine birds. In the nest box breeding population of Coal Tits (Periparus ater), EPO had a higher fecundity in subsequent years than their half-siblings when offspring had originated from late broods (Schmoll et al., 2005). In addition, in Common Yellowthroats (Geothlypis trichas), the T-cell-mediated immune response of EPO were stronger than that of WPO, and this was detected in the colder breeding season (Garvin et al., 2006). Similarly, Blue Tit (Cyanistes caeruleus) EPO were more likely to produce a stronger humoral immune response in comparison to WPO in experimentally enlarged broods (Arct et al., 2013). Moreover, EPO were larger, longer-winged and heavier than WPO in Tree Swallows (Tachycineta bicolor), and this phenomenon was most pronounced under heightened predation risk (Hallinger et al., 2020). These studies indicated that EPO could be better equipped with higher fitness than WPO to mitigate environmental stress. Therefore, the differences between WPO and EPO could most readily be observed when environments are relatively unfavorable.
Long-term environmental heavy metal pollution presents one such unfavorable condition, and it can potentially devastate the environment as well as wildlife. For example, heavy metal contamination can result in poor body condition by influencing metabolic adaptation and responses to stressors in Guillemots (Uria aalge) (Debacker et al., 2000) and Great Tits (Parus major) (Janssens et al., 2003). In addition, nest survival rate of Northern Mockingbirds (Mimus polyglottos) was lower in the higher lead neighborhood (Hitt et al., 2023). Indeed, heavy metal pollution leads to strong selection on fitness-related traits in wild birds. Interestingly, relatively fast recovery of reproductive parameters in the Pied Flycatcher (Ficedula hypoleuca) was found in response to decreasing heavy metal emissions (Eeva and Lehikoinen, 2015), suggesting that birds can exhibit a powerful response to the threat of heavy metal pollution. If EPO are better than WPO at managing unfavorable environments, the differences should become apparent under environmental heavy metal pollution. However, to date, there have been limited research efforts into investigating EPP in heavy metal pollution-influenced wild bird populations.
It is predicted that if long-term heavy metal pollution dampens the growth of offspring (Ding et al., 2020; Hitt et al., 2023), then females will pursue superior males as extra-pair mates to compensate for lowered offspring viability according to the “good gene hypothesis”. In addition, heavy metal pollution could reduce the population size, consequently raising the degree of inbreeding (Yang et al., 2020a). Then, female could select males with lower genetic similarity as extra-pair mates to increase the heterozygosity of offspring according to the “genetic compatibility hypothesis”. Accordingly, if female genetic benefits from EPP depends on the environments, females could bias the sex of EPO towards male under environmental heavy metal pollution according to the “sex-allocation theory” (Johnson et al., 2009). In this study, we firstly compared the body size, sex ratios and heterozygosity of Tree Sparrow (Passer montanus) offspring originating from mixed paternity nests that experienced different environments (as represented by long-term heavy metal pollution) to explore the variation of female genetic benefits from EPP. Moreover, we also investigated the body size, heterozygosity and relatedness of social mates and extra-pair mates of females to probe the response of female extra-pair mate choice to environmental heavy metal pollution.
The experiments were approved by the Ethical Committee of Animal Experiments of School of Life Sciences, Lanzhou University.
Two field sites, 110 km apart, in Gansu Province, NW China with varying degrees of heavy metal pollution were selected for sampling. Baiyin (BY) has been polluted mainly by heavy metals such as copper (Cu), zinc (Zn), lead (Pb) and cadmium (Cd) over the past 60 years due to its mining and smelting industry (Liu et al., 2017). Since the local mine resources have decreased dramatically, it was listed in one of the first batch of resource-exhausted transformation cities of China in 2008. Although the area has become resource-exhausted, industrial waste seriously polluted local soil, irrigating water and crops (Li et al., 2006; Liu et al., 2016; Ai et al., 2018). The current study selected Liangzhuang village (104°23′ E, 36°26′ N) in the BY area as an experimental site. We also selected Weijiachuan village (103°15′ E, 35°56′ N) in Liujiaxia (LJX), an area with little industry activity as a reference site. Heavy metal accumulation in the soil from the two areas was shown in Appendix Table S1.
The Tree Sparrow is a resident songbird whose breeding season usually lasts from the beginning of April to the end of July in the two areas. Incubation and feeding of offspring are undertaken by both parents, and clutch size ranges from three to six eggs (Ding et al., 2019). EPP is common in this socially monogamous species. Moreover, Tree Sparrows from BY (14.3%) displayed a higher EPP level than those from LJX (7.6%) (Yang et al., 2021). Higher heavy metal accumulation in the food sources (Appendix Table S2) and tissue (Appendix Table S3) of Tree Sparrows from BY was detected. Furthermore, we also found male Tree Sparrows with smaller body sizes and nestlings with lower fledging rate from BY than those from LJX (Ding et al., 2020; Yang et al., 2020b).
The nest box populations of Tree Sparrows were studied from 2017 to 2019 during the breeding seasons in BY and LJX. All nest boxes were checked weekly from the beginning of April of each year. The nest boxes with nest materials were checked daily to determine the clutch initiation data and exact hatching data. After hatching, the nestlings were immediately tagged by trimming the nails. Parent birds (defined as those provided parental care) were trapped using mist nets in the nestling period (7th to 9th days). Although Tree Sparrows are sexually monomorphic, the sex of parent birds was distinguished in the breeding seasons based on incubation patches (Selander and Yang, 1966). To obtain DNA for paternity analysis, a small amount of blood (10–20 μL) was sampled using brachial venipuncture from parent birds upon capture and from all nestlings after the 8th day of the nestling period. All blood samples were anti-coagulated and stored in 1.5 mL centrifuge tubes with absolute ethanol. We recorded the mass of nestlings using a digital balance (to the nearest 0.01 g) and measured two size metrics (tarsus and wing length) using a digital caliper (to the nearest 0.01 mm) daily until they fledged or died. The mass and two size metrics of parent birds were also measured. Subsequently, they were ringed with uniquely numbered color-rings for identification.
The QIAGEN DNeasy Blood and Tissue Kit was used to extract genomic DNA from blood samples of all individuals. Two multiplex PCR systems containing seven microsatellites were employed for paternity analysis. If the nestlings have two or more mismatched loci between social males and offspring, these nestlings were considered as EPO (confidence level = 95%), and detailed description of the methods is presented in Appendix Table S4. The paternity analysis dataset was published in our previous study (Yang et al., 2021). There were 91 nests (BY: 48 nests; LJX: 43 nests) that contained 351 nestlings (BY: 181 nestlings; LJX: 170 nestlings), and detailed information was shown in Appendix Table S5. In these nests, 28 mixed paternity nests (BY: 17 nests; LJX: 11 nests) that contained 39 EPO (BY: 26 nestlings; LJX: 13 nestlings) and 66 WPO (BY: 39 nestlings; LJX: 27 nestlings) were identified, and EPO from 13 nests (BY: 7 nests; LJX: 6 nests) were able to be assigned a genetic father (extra-pair male).
Owing to significant correlations of mass, tarsus and wing length (r: 0.525–0.653; all p < 0.05), we implemented principal component analysis (PCA) and used the scores of PC1 that explained 72.591% of the variation (loadings: mass: 0.881, tarsus length: 0.854, wing length: 0.820) to evaluate body size of adults, with superior-quality individuals possessing a higher body size index.
To measure the heterozygosity of all individuals on an identical scale, the standardized heterozygosity (SH) was used where the proportion of heterozygous typed loci in an individual was divided by the mean observed heterozygosity of typed loci (Coltman et al., 1999).
To prepare for the fledging stage, the nestlings begin to lose mass after the 10th day of the nestling period (Ding et al., 2020). Consequently, we used the scores of PC1 that explained 63.406% of the variation (loadings: mass: 0.874, tarsus length: 0.702, wing length: 0.803) on this day to evaluate body size of nestlings.
The heterozygosity of EPO and WPO was analyzed using the same method as social males and extra-pair males in the part of 2.3.3.2.
The P2/P8 is a sexing primer-pair in birds. To discriminate the sex of nestlings, PCR program was performed based on a previous study (Griffiths et al., 1998). Furthermore, 6% Native-PAGE was used to separate PCR products, and males and females exhibit one band and two bands, respectively.
Queller&Goodnight’s estimator (rQG) was used as an effective indicator to assess genetic relatedness of mate pairs based on the seven microsatellite loci (Appendix Table S4) in Kingroup 2.0 software (Konovalov et al., 2004). Other data analysis was conducted in the SPSS 22.0 statistical package program (IBM SPSS Inc., USA). The normal distribution of the data was checked using the Kolmogorov–Smirnov test, and homogeneity of variance was examined using Levene’s test. We compared: 1) the body size and heterozygosity between extra-pair males and social males; and 2) the length of tarsus and wing between EPO and WPO; and 3) the body size and heterozygosity between males from BY and LJX (using independent samples t-tests). Because relatedness data were non-normally distributed, the Mann–Whitney U test was employed. Growth rate equations of tarsus and wing length of nestlings were determined by nonlinear regression analysis. The sex ratios of EPO, WPO and total offspring (TO) were analyzed using a binomial test. The linear mixed model (restricted maximum likelihood (REML) estimation) was used to analyze the heterozygosity and body size of WPO and EPO. Populations (two levels, BY and LJX), paternity (two levels, WPO and EPO), interaction between population and paternity, offspring sex, year of study were set as fixed factors, and the nest was set as random factor. The level of significance of all statistical tests was p < 0.05. All values are expressed as the mean ± SD. The bar graph, line chart and scatter plots were drawn using OriginPro 9.0 (OriginLab, USA).
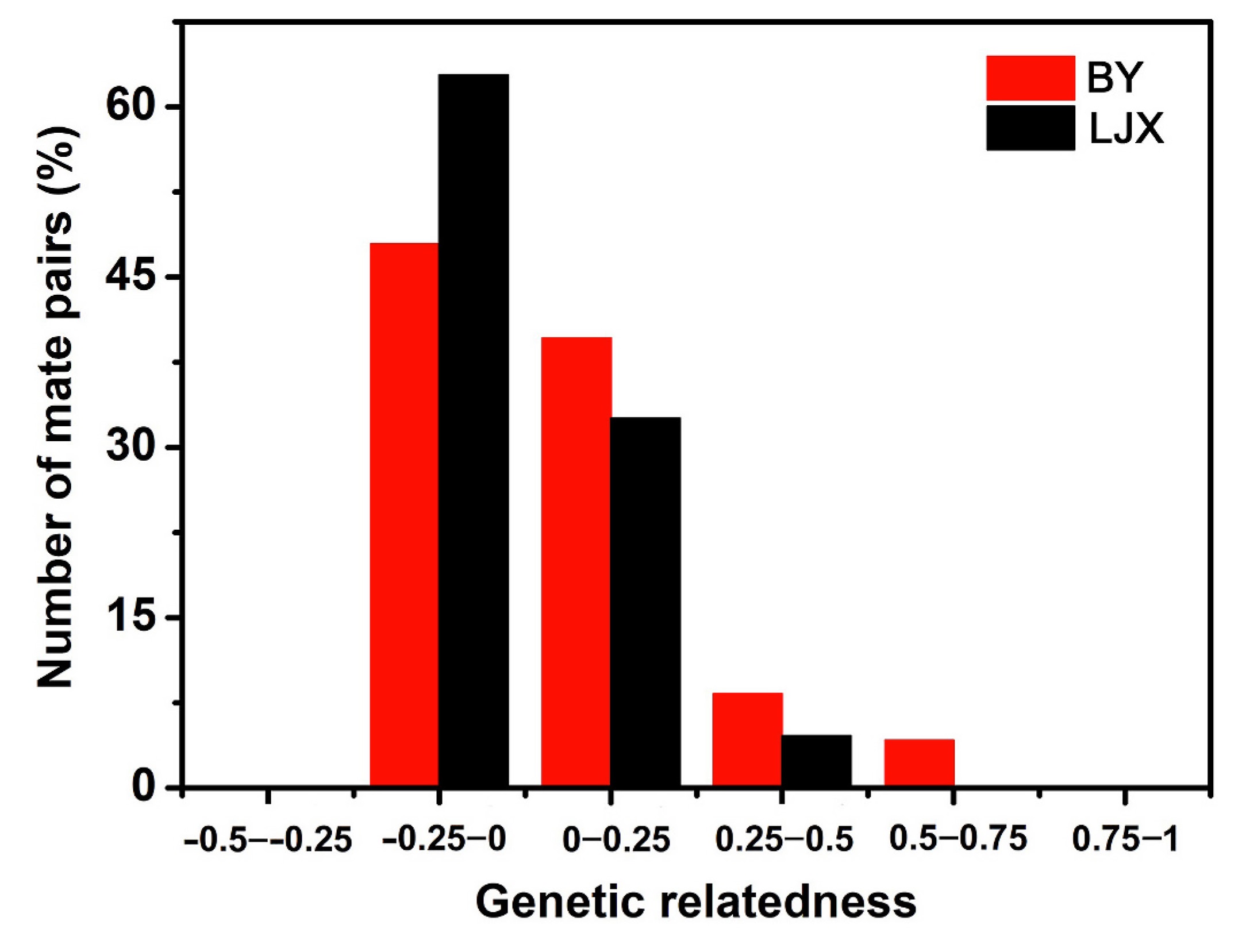
The average relatedness of social mates from the BY Tree Sparrow population did not differ significantly from that of the LJX Tree Sparrow population (BY: 0.073 ± 0.156, −0.087 to 0.645; LJX: 0.025 ± 0.100, −0.011 to 0.316; Mann-Whitney U test, Z = −1.499, p = 0.134, N = 91). Social mates’ relatedness greater than 0.5 occurred in the BY Tree Sparrow population, but this phenomenon was not found in the LJX Tree Sparrow population (Fig. 1). Furthermore, females were genetically more similar to social males than to extra-pair males in the BY Tree Sparrow population, and the difference was close to significant (social males: 0.185 ± 0.196; extra-pair males: 0.026 ± 0.076; Mann-Whitney U test, Z = 1.853, p = 0.073, N = 14). However, there was no significant difference between the relatedness of social mates and extra-pair mates in the LJX Tree Sparrow population (social males: 0.099 ± 0.086; extra-pair males: 0.061 ± 0.032; Mann-Whitney U test, Z = 0.641, p = 0.589, N = 12).
In the BY Tree Sparrow population, the body size score of extra-pair males was significantly greater than that of social males (extra-pair males: 0.17 ± 0.81; social males: −1.22 ± 0.71; t12 = 3.433, p = 0.005, N = 14). However, no significant difference in body size score between extra-pair males and social males from the LJX Tree Sparrow population was recorded (extra-pair males: 0.83 ± 0.32; social males: 0.40 ± 0.59; t10 = 1.549, p = 0.152, N = 12).
No significant difference in heterozygosity existed between extra-pair males and social males from the BY Tree Sparrow population (extra-pair males: 0.9491 ± 0.1162; social males: 0.8305 ± 0.1776; t12 = −1.369, p = 0.196, N = 14). Similar phenomena were also found in the LJX Tree Sparrow population (extra-pair males: 0.9460 ± 0.1114; social males: 0.8919 ± 0.1553; t10 = −0.632, p = 0.541, N = 12).
EPO had greater body size score than WPO in the linear mixed model using all mixed paternity nests from BY and LJX, and there was a significant interaction between population and paternity (Table 1). In addition, EPO had a greater body size score than WPO in BY (EPO: 0.21 ± 0.62; WPO: −1.06 ± 0.62; t50 = 7.371, p < 0.001, N = 52), while there was no difference between EPO and WPO in LJX (EPO: 0.74 ± 0.65; WPO: 0.63 ± 0.78; t35 = 0.428, p = 0.671, N = 37).
| Source of variation | df | F | p |
| Intercept | 1,20.7 | 2.89 | 0.104 |
| Year | 2,23.3 | 1.03 | 0.373 |
| Sex | 1,72.8 | 0.01 | 0.983 |
| Population | 1,38.1 | 35.1 | <0.001 |
| Paternity | 1,62.2 | 24.1 | <0.001 |
| Population × Paternity | 1,63.1 | 18.6 | <0.001 |
A significant interaction between population and paternity in heterozygosity from the linear mixed model was observed (Table 2). EPO were significantly more heterozygous than WPO in mixed paternity nests from the BY population (EPO: 0.95 ± 0.14; WPO: 0.84 ± 0.18; t63 = 0.428, p = 0.019, N = 65). However, there was no significant difference in heterozygosity between EPO and WPO in mixed paternity nests from the LJX population (EPO: 0.91 ± 0.10; WPO: 0.94 ± 0.16; t38 = 0.719, p = 0.476, N = 40).
| Source of variation | df | F | p |
| Intercept | 1,13.7 | 2385.15 | <0.001 |
| Year | 2,35.9 | 0.44 | 0.645 |
| Sex | 1,90.9 | 0.82 | 0.367 |
| Population | 1,72.2 | 1.07 | 0.305 |
| Paternity | 1,88.4 | 1.36 | 0.247 |
| Population × Paternity | 1,84.1 | 4.08 | 0.047 |
Both the tarsus and wing length of nestlings followed the logistic growth curve (Appendix Table S6). EPO from the BY population had significantly longer tarsus and wing length than those of WPO, while no significant difference between EPO and WPO was found from the LJX population (Fig. 2). In addition, the sex ratios of EPO (BY: p = 0.846, N = 26; LJX: p = 0.581, N = 13), WPO (BY: p = 0.630, N = 155; LJX: p = 0.750, N = 157) and TO (BY: p = 0.552, N = 181; LJX: p = 0.591, N = 170) from the two Tree Sparrow populations did not deviate significantly from 1:1 (Fig. 3).
In the Tree Sparrow population exposed to long-term environmental heavy metal pollution, EPO significantly outperformed WPO in body size and heterozygosity. However, the differences between EPO and WPO were not significant in the Tree Sparrow population from the relatively unpolluted area. Furthermore, we also found that the females selected males with lower genetic similarity and greater body size as extra-pair mates only under environmental heavy metal pollution, which suggested that sexual selection of females may vary across environmental conditions. Therefore, our results were consistent with the “context-dependence hypothesis” that females should plastically adjust extra-pair mate choice depending on the environmental context, thereby gaining more genetic benefits from EPP when environmental conditions are relatively unfavorable (Schmoll, 2011).
Body size is important for individual survival and reproduction (Graveland, 1998). In this study, extra-pair males had a higher body size index than social males from the heavy metal polluted area (BY), but this phenomenon was not found in the relatively unpolluted area (LJX). Theory suggests that if environmental conditions affect offspring performance, females could be likely to show phenotypic plasticity in mating strategies (Qvarnström et al., 2000; Chaine and Lyon, 2008). According to our previous study, the body parameters of Tree Sparrow nestlings would be reduced under environmental heavy metal pollution (Ding et al., 2020). The “good gene hypothesis” proposed that females could acquire good genes for their offspring from extra-pair males (Kempenaers et al., 1992; Brouwer and Griffith, 2019). Specifically, females might select extra-pair males to make EPO inherit specific genes, which suggests that the differences in offspring traits arise as a result of additive genetic variances (Hallinger et al., 2020). In line with this hypothesis, we found that EPO had larger body size than WPO in the Tree Sparrow population from BY. However, there was no significant difference in the body size between EPO and WPO from LJX. Therefore, it is possible that females sought males with larger body size as extra-pair mates to produce higher quality of offspring under relatively unfavorable conditions. Other explanations are, however, possible. For example, all offspring could exhibit higher quality regardless of the quality of the father in favorable environmental conditions, and therefore there was no significant difference between EPO and WPO in these environments.
Females are biasing offspring sex based on paternity according to “sex-allocation theory” (Johnson et al., 2009). However, we found the sex ratio of EPO in the two Tree Sparrow populations was not biased toward males. On one hand, female may have limited ability to manipulate offspring sex, given the chromosomal nature of sex determination in birds (West and Sheldon, 2002). On the other hand, the net selection pressure on females could reduce the male ratio of EPO that we anticipated (Johnson et al., 2009). Moreover, in a Coal Tit (Parus ater) population under natural condition, there was also no indication of sex ratio bias with paternity (Dietrich-Bischoff et al., 2006).
The relatedness among individuals can be used to evaluate the level of inbreeding within populations (Kruuk et al., 2002). In the present study, social mates’ relatedness exceeding 0.5 occurred in the BY Tree Sparrow population. The extreme inbreeding phenomenon of social mates could increase the probability of homozygosity for the loci, which in turn reduces heterozygosity of offspring (Saccheri et al., 1998). Our previous study found that the genetic diversity level of Tree Sparrow populations decreased as the concentrations of heavy metal increased in this area (Yang et al., 2020a). The heterozygosity of individuals is closely related to their fitness and survival (Hansson and Westerberg, 2002; Zeng et al., 2017). High individual heterozygosity not only reduces the expression of recessive deleterious alleles but also increases the amount of useful gene products (Brown, 1997). Interestingly, we found that female Tree Sparrows were inclined to select males with lower genetic similarity instead of males with higher heterozygosity as extra-pair mates, and the average heterozygosity of EPO was higher than that of WPO in BY, supporting the “genetic compatibility hypothesis”. A previous study also discovered that EPO were more heterozygous than WPO in Tree Swallow population (Stapleton et al., 2007). However, no significant difference in heterozygosity between EPO and WPO from LJX was observed in this study, which further confirmed that EPP could be strongly affected by environmental conditions.
EPP of Tree Sparrows showed an adaptive response to long-term environmental heavy metal pollution. Correspondingly, variation in extra-pair mate choice can increase female genetic benefits, however, the mating strategy of female Tree Sparrows could require high energy expenditure to search for extra-pair mates, thereby causing adverse effects on their self-maintenance under long-term environmental heavy metal pollution. As a consequence, a trade-off between self-maintenance and reproductive efforts of females can be used to elucidate the variation in EPP under chronic environmental heavy metal pollution (Zera and Harshman, 2001; Zhang et al., 2020). Furthermore, both the “good gene hypothesis” and “genetic compatibility hypothesis” were important for explaining the variation of EPP in the Tree Sparrow population exposed heavy metal pollution. Such simultaneous extra-pair mate choice could be mediated through an evolutionarily stable strategy where females simultaneously optimize their choice for good genes and compatible genes (Neff and Pitcher, 2005). However, other factors such as temperatures, food availability, breeding density, breeding synchrony, the level of urbanization etc., may be also influential for the determinants of EPP (Garvin et al., 2006; Di Lecce et al., 2023). We found there was no significant difference between the two study areas in the temperatures of Tree Sparrow breeding season (Appendix Table S7). The diets of Tree Sparrows from the two populations are similar, mainly including plant food (cereal grains and grass seeds) and some invertebrates (Ai et al., 2019). There was no significant correlation between the EPP and breeding density and synchrony of Tree Sparrows from the two populations (Appendix Table S8). Furthermore, we found there was no significant difference in the urbanization level between BY and LJX (Appendix Fig. S1). Therefore, long-term heavy metal pollution, as one of the key environmental variables, could have potentially far-reaching implications on the mating preferences and tactics of female Tree Sparrows.
Female birds usually pursue EPP to gain potential genetic benefits, which could be vulnerable to environmental changes. When the Tree Sparrow population was exposed to long-term environmental heavy metal pollution, females preferred the extra-pair males with larger body size and lower genetic similarity, and EPO had higher heterozygosity and bigger body size than WPO. However, these phenomena were not observed under relatively unpolluted conditions. The results of the current study therefore confirmed preliminarily that EPP was context-dependent within the natural population of the socially monogamous Tree Sparrows, and female genetic benefits from EPP are likely to become detectable under unfavorable environmental conditions. Therefore, long-term environmental heavy metal pollution as a selective pressure may have shaped the mating behavior of females and further promoted the evolution of alternative reproductive strategies in passerine birds. Although the environmental heavy metal pollution is gradually decreasing, the remaining environmental problems are still serious. The avian research in the polluted areas is helpful to evaluate the local ecological restoration situation.
Wenzhi Yang: Writing – review & editing, Writing – original draft, Investigation, Data curation, Conceptualization. Yue Shen: Writing – review & editing, Investigation. Yuquan Miao: Writing – review & editing. Zhaocun Lin: Writing – review & editing, Investigation. Yingmei Zhang: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We would like to thank the Core Facility of School of Life Sciences and the Supercomputing Centre of Lanzhou University for their support.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2024.100203.
| 1. | Xudong Li, Jiangping Yu, Li Zhang, et al. Nest site selection during the second breeding attempt in Japanese tits (Parus minor): effects of nest site characteristics. Scientific Reports, 2025, 15(1) DOI:10.1038/s41598-025-87928-2 |
| 2. | Xin Luo, Shuai Gao, Sichun Tong, et al. Non-Breeding Season Habitat Selection of Three Commonly Occurring Bird Species in a Patchy Habitat in SE China. Land, 2024, 13(6): 807. DOI:10.3390/land13060807 |
| 3. | Ning Li, Ning Tang, Yuanhao Ren, et al. Effects of forest ropeway construction on bird diversity and its seed dispersal mutualism for endangered Taxus chinensis, southeast China. Global Ecology and Conservation, 2022, 38: e02227. DOI:10.1016/j.gecco.2022.e02227 |
| 4. | Alessandro Berlusconi, Alessio Martinoli, Lucas A. Wauters, et al. Year-round multi-scale habitat selection by Crested Tit (Lophophanes cristatus) in lowland mixed forests (northern Italy). Avian Research, 2022, 13: 100058. DOI:10.1016/j.avrs.2022.100058 |
| 5. | Zheng Wang, Shuai Gao, Xinglong Huang, et al. Functional importance of bird-dispersed habitat for the early recruitment of Taxus chinensis in a fragmented forest. Acta Oecologica, 2022, 114: 103819. DOI:10.1016/j.actao.2022.103819 |
| 6. | Evolution of the Arborescent Gymnosperms. DOI:10.1017/9781009262965.017 |
| Source of variation | df | F | p |
| Intercept | 1,20.7 | 2.89 | 0.104 |
| Year | 2,23.3 | 1.03 | 0.373 |
| Sex | 1,72.8 | 0.01 | 0.983 |
| Population | 1,38.1 | 35.1 | <0.001 |
| Paternity | 1,62.2 | 24.1 | <0.001 |
| Population × Paternity | 1,63.1 | 18.6 | <0.001 |
| Source of variation | df | F | p |
| Intercept | 1,13.7 | 2385.15 | <0.001 |
| Year | 2,35.9 | 0.44 | 0.645 |
| Sex | 1,90.9 | 0.82 | 0.367 |
| Population | 1,72.2 | 1.07 | 0.305 |
| Paternity | 1,88.4 | 1.36 | 0.247 |
| Population × Paternity | 1,84.1 | 4.08 | 0.047 |