| Citation: | Peng LI, Ping DING, Kenneth J. FEELEY, Jingcheng ZHANG, Pingping JIANG. 2010: Patterns of species diversity and functional diversity of breeding birds in Hangzhou across an urbanization gradient. Avian Research, 1(1): 1-8. DOI: 10.5122/cbirds.2009.0020 |
Given the rapid rise in human population and increasing urbanization,it is important to understand their potential impacts on biodiversity. From March 2007 to August 2007, we conducted bird surveys in 90 strip transects,each 3 km long and 100 m wide,along a gradient of urbanization in Hangzhou,China. This gradient spanned a range of urbanization levels including urban areas,rural-urban continuum areas,farming areas,mixed forest/farming areas and forested areas. We recorded 96 breeding bird species and classified them into nine functional groups based on nesting requirements. The nine functional groups consisted of canopy nesters,shrub nesters,canopy/shrub nesters,natural cavity nesters,building nesters,natural cavity/building nesters,ground nesters,water surface nesters and parasitic nesters. Species and functional diversities were estimated based on the Shannon-Wiener index. Environmental data of each transect as human disturbance,vegetation cover and building index were also measured,and a synthetic urbanization index of each transect was introduced based on these data. We used regression analyses to model the relationship of species abundance,species diversity,functional abundance and functional diversity with this synthetic index. The results show that urbanization significantly reduces species richness,species diversity,functional richness and functional diversity,but the specific patterns differed. The relationship between species abundance/species diversity and urbanization is linear. In contrast,the relationship between functional diversity and urbanization was quadratic. In other words,with increased urbanization,functional diversity declined only slightly at first but then dropped at an accelerating rate. This implies that,although moderate urbanization reduces species diversity of breeding birds,it affects functional diversity of breeding birds only slightly in Hangzhou. The regression analysis of species diversity and functional diversity suggests a quadratic relationship between species diversity and functional diversity,i.e.,a linear relationship between species diversity and functional diversity can only exist at low diversity levels across urbanization gradients and increasing species abundance does not lead to an increase in functional diversity at the highest diversity levels.
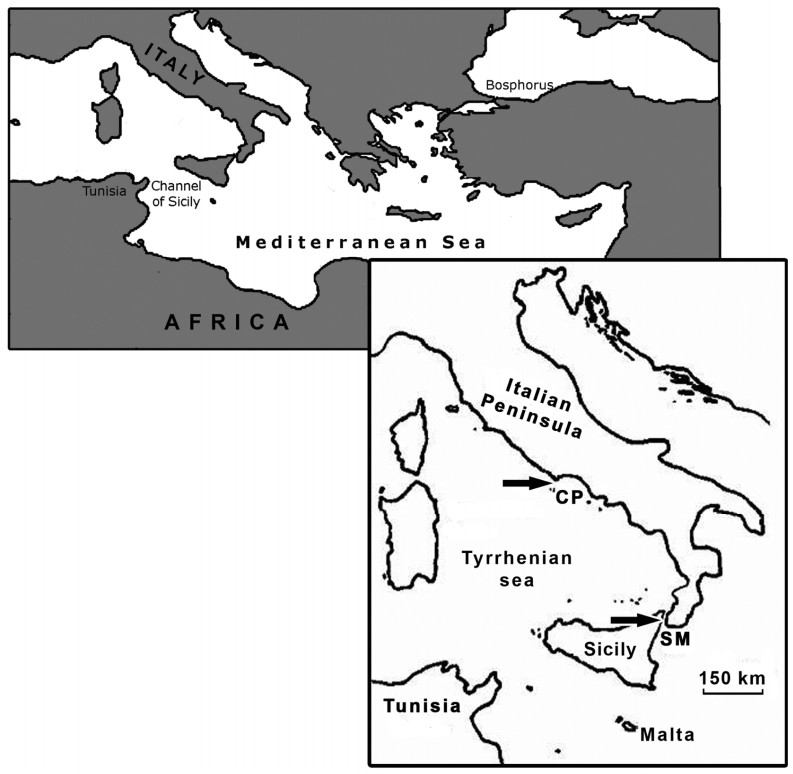
During migration Accipitriformes use mostly soaring flight optimizing the use of thermal currents, avoiding long water crossings to limit powered flight over water in order to reduce energetic costs (Kerlinger, 1989). Several factors influence the decision whether to cross the sea or not and shape the path during the crossing: weather conditions, geography, physiological state of the birds, flocking behavior, time of the day and experience (age dependent), while the risk of mortality probably increases with the absolute distance of the crossing (Kerlinger, 1989; Bildstein, 2006). As well, the flight morphology plays a role; in particular, species with relatively long wings (high aspect ratio = high ratio of wing span squared to wing area; see Kerlinger, 1989) are more suited to undertake crossings of large bodies of water since this feature decreases the induced drag and thus the energy required for powered flight (Kerlinger, 1985). The Western Marsh Harrier (Circus aeruginosus), for instance, is able to cross large bodies of water having high aspect ratio wings; both adults and juveniles migrate on a broad front across the Mediterranean basin (Agostini, 2001; Agostini et al., 2001, 2003; Panuccio et al., 2002). Conversely, in the European Honey Buzzard (Pernis apivorus), a species with a lower wing aspect ratio than the Western Marsh Harrier, the water crossing tendency is age dependent (Agostini and Logozzo, 1995; Agostini et al., 2002; Schmid, 2000; Hake et al., 2003). In particular, during autumn movements through the Central Mediterranean region, thousands of adult (experienced) birds follow the Italian Peninsula avoiding the crossing of the Tyrrhenian Sea (Agostini and Logozzo, 1997; Panuccio et al., 2005). Upon reaching the Strait of Messina between the "toe" of southern Italy and Sicily, they turn west. They will fly across Sicily and then southwest across the Channel of Sicily, the shortest crossing of the Central Mediterranean (approx. 150 km), heading towards Tunisia (Agostini et al., 2000, 2005b; Fig. 1). In contrast, juveniles show a broader front of migration over the sea such as the Western Marsh Harriers. In particular juvenile European Honey Buzzards, migrating later than adults, cannot learn the shortest routes to cross the sea by following experienced birds and tend to move along an innate NE-SW axis between breeding and wintering areas (Agostini and Logozzo, 1995; Agostini et al., 2002; Agostini, 2004). The aim of our study was to compare the water crossing behavior of these two species in relation to wind conditions, the time of the day and the age of migrants, carrying out visual observations at the Circeo Promontory, a watchsite in central Italy. At this site, hundreds of Western Marsh Harriers, mostly adults (Agostini et al., 2001, 2003) and hundreds of European Honey Buzzards, largely juveniles (Corbi et al., 1999; Agostini et al., 2002, 2004), concentrate each autumn.
The Circeo Promontory (41°12′N, 13°03′E) is located at the southernmost point of the Pianura Pontina, reaching 541 m a.s.l. and approximately 500 km northeast of Tunisia (Fig. 1). We used a post (altitude approx. 400 m) in a military zone, on the roof of the ENAV (Ente Nazionale Assistenza al Volo) building; from this post it was possible to observe the flight behavior of birds both inland and over the sea. The Ponziane Islands are nearly always visible from this watch-point; the closer island is Zannone, about 30 km south-southwest of the promontory. A total of 324 hours of observations were carried out between 26 August and 30 September 2002, the peak migration period of these two species, from 09:00 until dusk, aided with binoculars and a telescope. Observations were interrupted only due to rain. As in previous studies investigating the water crossing behavior of these species in relation to the time of the day (Panuccio et al., 2002; Agostini et al., 2005a), each day was divided into three periods (solar time): morning (09:00–11:59), midday (12:00–14:59) and afternoon (15:00–18:00). Meteorological data concerning wind direction were provided by the Meteorological Station of Latina and are available on the website www.ilmeteo.it/dati.htm. The maximum time between the passage of raptors and the meteorological data check was half an hour. Meteorological data were not available for 36 hours. In the analysis we considered crossing and not crossing birds (birds roosting at the site, flying back inland or flying along the coast) using flocks and birds migrating singly as sampling units to evaluate whether the direction of wind and the time of the day affected the choice of these species to undertake the sea crossing or not. Finally, among birds stopping their migration, we did not consider those flying along the coast, but only those roosting at the site and those flying back inland.
It was possible to follow the movements of 371 flocks (European Honey Buzzard n = 212; Western Marsh Harrier n = 159) and 340 birds migrating singly (European Honey Buzzard n = 180; Western Marsh Harrier n = 160). Only 16 (4.3%) flocks (12 among European Honey Buzzards and 4 among Western Marsh Harriers) and 8 (2.4%) solitary birds (4 European Honey Buzzards and 4 Western Marsh Harriers) left the site flying along the coast. Considering birds that stopped migration (birds roosting at the site or flying back inland) and undertook the crossing of the Tyrrhenian Sea, the behavior of European Honey Buzzards and Western Marsh Harriers did not differ significantly among both flocks (Table 1a; contingency table: χ2 = 1.97, df = 1, p > 0.05) and solitary birds (Table 1b; χ2 = 0.47, df = 1, p > 0.05). During the observation period, tail winds were almost never recorded (11 hours; 3.4%). European Honey Buzzards were not affected by wind conditions when migrating in flocks (Table 2a; contingency table: χ2 = 3.22, df = 2, p > 0.05) while solitary individuals undertook the water crossing rather than stopping migration during the absence of wind and vice versa during head winds (Table 2b; χ2 = 8.15, df = 2, p < 0.05). In contrast, Western Marsh Harriers were affected by wind conditions when migrating both in flocks (Table 2c; χ2 = 7.8, df = 2, p < 0.05) and singly (Table 2d; χ2 = 7.88, df = 2, p < 0.05) with the lowest proportion of birds seen stopping migration during conditions of no wind. Both European Honey Buzzards and Western Marsh Harriers showed the same behavior during all times of the day. In particular, considering both flocks and solitary birds, they undertook the sea crossing rather than stopping migration during the morning. The opposite was true in the afternoon (Table 3a: χ2 = 37.29, df = 2, p < 0.01; Table 3b: χ2 = 19.53, df = 2, p < 0.01; Table 3c: χ2 = 30.65, df = 2, p < 0.01; Table 3d: χ2 = 36.22, df = 2, p < 0.01). It is interesting to note that no wind conditions prevailed during the morning, while the occurrence of head winds was higher at midday and in the afternoon (Table 4; χ2 = 56.94, df = 4, p < 0.001). When comparing the average flock sizes of the two species, the difference was statistically significant between flocks stopping migration (EHB = 4.3 ± 0.43 [SE]; WMH = 7.1 ± 1.5 [SE]; z = 4.67, p < 0.01) but not between those undertaking the crossing (EHB = 5.5 ± 0.45 [SE]; WMH = 4.3 ± 0.37 [SE]). Larger flocks of Western Marsh Harriers formed in the afternoon when they roosted on trees at the site (see also Panuccio and Agostini, 2006). Our results confirm that flocking significantly affects the decision (crossing or not) of European Honey Buzzards when facing a water barrier (Agostini et al., 1994, 2005b) since, unlike flocks, single birds were less inclined to undertake the crossing, hesitating during head winds. Conversely, among Western Marsh Harriers, solitary birds behaved much the same as those flying in flocks. Thus, European Honey Buzzards behaved as reported for raptors that have a higher wing aspect ratio. They were even more inclined than Western Marsh Harriers to undertake the crossing of the water barrier when migrating in flocks. It was possible to determine the age of 1019 birds by observation of their plumage (Forsman, 1999). As reported in a previous study made at this watchsite (Corbi et al., 1999), juveniles (n = 429) outnumbered adults (n = 169) among European Honey Buzzards. Among Western Marsh Harriers, adults (n = 309) outnumbered juveniles (n = 112). When considering the behavior of migrants (crossing; stopping; flying along the coast) in relation to the two age classes, adults behaved like juveniles in both species (Table 5a; contingency table: χ2 = 0.51, df = 2, p > 0.05; Table 5b: χ2 = 3.82, df = 2, p > 0.05). This result was expected in the case of the Western Marsh Harrier since, as mentioned earlier, this is a species which is more adapted to make crossing flights. In contrast, adult European Honey Buzzards, unlike juveniles, were expected to fly along the coast and, as mentioned already, crossing the sea at narrower points (the Strait of Messina and the Channel of Sicily; see also Agostini and Panuccio, 2005) and able to orientate themselves using their navigational abilities and compensating for wind drift (Meyer et al., 2000; Thorup et al., 2003; Agostini et al., 2005b). Our observations suggest that adults passing at the Circeo Promontory each autumn are probably younger, less experienced birds. Similar to the Circeo Promontory, a scarce passage of "adult" European Honey Buzzards was reported over the islands of Malta, Pianosa and Cabrera (Balearic Islands), where mostly juveniles concentrated during autumn migration (Rebassa, 1995; Agostini et al., 2002; Paesani and Politi, 2002). Perhaps, as suggested by Agostini et al.(2002, 2004), juvenile European Honey Buzzards passing over the Circeo Promontory do not have enough experience about the high energetic costs of long powered flight over water and, as younger adults, they do not know the shortest flyway to cross the Central Mediterranean, between western Sicily and Tunisia. Probably for this reason, both juvenile and adult European Honey Buzzards observed in our study behaved similarly, showing a strong tendency to continue migration over the sea. In contrast, our observations do not confirm the hypothesis that the difference in migration strategy between adults and juveniles in this species may partly depend on differences in the timing of migration (Schmid, 2000). In particular, Schmid suggested that since adults mainly migrate across Europe in late August to early September, when it is still possible to travel by soaring flight to a high degree, they use a safer overland flight, since this is compensated by low energetic costs of soaring, compared to flapping flights. Juveniles migrate about two to three weeks later, when meteorological thermal models suggest that conditions for using soaring flights are poorer. Thus, Schmid concluded that they may as well use flapping flights, choosing the shortest way to wintering grounds in West Africa. However, during our study, European Honey Buzzards behaved in the same way during the entire period and, as expected, adults migrated earlier in the season (see also Agostini et al., 2004).
| Crossing behavior |
Flocks (a) | Solitary individuals (b) | |||
| EHB | WMH | EHB | WMH | ||
| Crossing | 128 (64%) | 87 (56%) | 107 (61%) | 88 (56.4%) | |
| Stopping | 72 (36%) | 68 (44%) | 69 (39%) | 68 (43.6%) | |
| Wind condition |
European Honey Buzzards | Western Marsh Harriers | |||||||||
| Flocks (a) | Solitary individuals (b) | Flocks (c) | Solitary individuals (d) | ||||||||
| Crossing | Stopping | Crossing | Stopping | Crossing | Stopping | Crossing | Stopping | ||||
| No wind | 34 (67%) | 17 (33%) | 33 (70%) | 14 (30%) | 19 (76%) | 6 (24%) | 41 (79%) | 11 (21%) | |||
| Head wind | 43 (58%) | 31 (42%) | 22 (42%) | 30 (58%) | 33 (44%) | 42 (56%) | 35 (57%) | 26 (43%) | |||
| Lateral wind | 15 (47%) | 17 (53%) | 15 (47%) | 17 (53%) | 23 (55%) | 19 (45%) | 14 (52%) | 13 (48%) | |||
| Time of the day |
European Honey Buzzards | Western Marsh Harriers | |||||||||
| Flocks (a) | Solitary individuals (b) | Flocks (c) | Solitary individuals (d) | ||||||||
| Crossing | Stopping | Crossing | Stopping | Crossing | Stopping | Crossing | Stopping | ||||
| Morning | 80 (77%) | 24 (23%) | 77 (74%) | 27 (26%) | 27 (96%) | 1 (4%) | 53 (91%) | 5 (9%) | |||
| Midday | 43 (61%) | 28 (39%) | 21 (46%) | 25 (54%) | 41 (59%) | 29 (41%) | 41 (57%) | 31 (43%) | |||
| Afternoon | 5 (20%) | 20 (80%) | 9 (35%) | 17 (65%) | 19 (33%) | 38 (67%) | 7 (27%) | 19 (73%) | |||
| Time of the day | No wind | Head wind | Lateral wind |
| Morning | 40 (61%) | 9 (14%) | 16 (25%) |
| Midday | 20 (19%) | 59 (55%) | 28 (26%) |
| Afternoon | 15 (14%) | 58 (55%) | 32 (31%) |
| Total | 75 (27%) | 126 (46%) | 76 (27%) |
| Age class | European Honey Buzzards (a) | Western Marsh Harriers (b) | |||||
| Crossing | Stopping | Flying along the coast |
Crossing | Stopping | Flying along the coast |
||
| Adults | 100 (59%) | 58 (34%) | 11 (7%) | 148 (48%) | 154 (50%) | 7 (2%) | |
| Juveniles | 238 (55%) | 157 (37%) | 34 (8%) | 66 (59%) | 43 (38%) | 3 (3%) | |
We are grateful to the Aeronautica Militare Italiana for permission to enter a military zone and the ENAV for the permission to use the observation post on the roof of its building. We wish to thank an anonymous referee for his useful comments to an earlier draft of the manuscript.
|
Beissinger SR, Osborne DR. 1982. Effects of urbanization on avian community organization. Condor, 8:75–83
|
|
Chen SH, Ding P, Zheng GM, Zhuge Y. 2000. Impacts of urbanization on the wetland water-bird communities in Hangzhou. Zool Res, 21(4):279–285 (in Chinese)
|
|
Chen FG, Luo SY. 1998. Fauna Sinica Aves Vol.9. Science Press, Beijing (in Chinese)
|
|
Cheng TH. 1978. Fauna Sinica Aves Vol.4. Science Press, Beijing (in Chinese)
|
|
Cheng TH. 1979. Fauna Sinica Aves Vol.2. Science Press, Beijing (in Chinese)
|
|
Cheng TH, Long ZY, Zheng BF. 1987. Fauna Sinica Aves Vol.11. Science Press, Beijing (in Chinese)
|
|
Cheng TH, Xian YH, Guan GX. 1991. Fauna Sinica Aves Vol.6. Science Press, Beijing (in Chinese)
|
|
Cheng TH, Long ZY, Lu TC. 1995. Fauna Sinica Aves Vol.10. Science Press, Beijing (in Chinese)
|
|
Cheng TH. 1997. Fauna Sinica Aves Vol.1. Science Press, Beijing (in Chinese)
|
|
Fu TS, Song YJ, Gao W. 1998. Fauna Sinica Aves Vol.14. Science Press, Beijing (in Chinese)
|
|
Hooper DU, Solan M, Symstad A, Díaz S, Gessner MO, Buchmann N, Degrange V, Grime P, Hulot F, Mermillod-blondin F, Roy J, Spehn E, van Peer L. 2002. Species diversity, functional diversity and ecosystem functioning. In: Loreau M, Naeem S, Inchausti P (eds) Biodiversity and Ecosystem Functioning: Syntheses and Perspectives. Oxford University Press, Oxford, pp 195–208
|
|
Li GY, Zheng BL, Liu GZ. 1982. Fauna Sinica Aves Vol.13. Science Press, Beijing (in Chinese)
|
|
Li PF, Li W, Zhou W, Wang CF, Cai HY. 2004. Ecological habits of bird of hawk family in Baishilazi region. J Liaoning Forest Sci Technol, 3:5–8 (in Chinese)
|
|
Melles S, Glenn S, Marti K. 2003. Urban bird diversity and landscape complexity: Species-environment associations along a multiscale habitat gradient. Conserv Ecol, 7(1):5
|
|
Moretti M, Bello F, Roberts SPM, Potts SG. 2009. Taxonomical vs. functional responses of bee communities to fire in two contrasting climatic regions. J Anim Ecol, 78:98–108
|
|
Naeem S. 2002. Autotrophic–heterotrophic interactions and their impacts on biodiversity and ecosystem functioning. In: Kinzig AP, Pacala SW, Tilman D (eds) The Functional Consequences of Biodiversity. Princeton University Press, Princeton, NJ, pp 120–150
|
|
Ricklefs RE. 2001. The Economy of Nature, 5th edn. W.H. Freeman and Company, New York
|
|
Rosenberg KV, Terrill SB, Rosenberg GH, 1987. Value of suburban habitats to desert riparian birds. Wilson Bull, 99: 643–654.
|
|
Sun MY, Li KQ, Zhu JJ, Kao CC, Sun XJ. Zhou ZS. 2002. Reproduction habits of three species of woodpeckers and their prey on insects. Forest Pest Dis, 21(2):12–14 (in Chinese)
|
|
Tilman D. 2001. Functional diversity. In: Levin SA (ed) Encyclopedia of Biodiversity, Vol 3. Academic Press, San Diego, CA, pp 109–120
|
|
Wang YP, Chen SH, Jing PP, Ding P. 2008. Black-billed Magpies (Pica pica) adjust nest characteristics to adapt to urbanization in Hangzhou, China. Can J Zool, 86:868–874
|
|
Zheng BL. 1985. Fauna Sinica Aves Vol.8. Science Press, Beijing (in Chinese)
|
|
Zheng GM. 1995. Ornithology. Beijing Normal University Press, Beijing (in Chinese)
|
|
Zheng GM, Zhang CZ. 2002. Birds in China. China Forestry Publishing House, Beijing (in Chinese)
|
| Sanjaya Weerakkody, Eben Goodale, Vimukthi R. Gunasekara, Yang Liu, Praveen Karanth, Sampath S. Seneviratne. 2023: Plumage assisted divergence in a vocally complex island endemic: The Dicrurus paradiseus species complex in Sri Lanka. Avian Research, 14(1): 100132. DOI: 10.1016/j.avrs.2023.100132 | |
| Yiwen Chen, Yat-tung Yu, Fanjuan Meng, Xueqin Deng, Lei Cao, Anthony David Fox. 2021: Migration routes, population status and important sites used by the globally threatened Black-faced Spoonbill (Platalea minor): a synthesis of surveys and tracking studies. Avian Research, 12(1): 74. DOI: 10.1186/s40657-021-00307-z | |
| Chuanwu Chen, Marcel Holyoak, Yanping Wang, Xingfeng Si, Ping Ding. 2019: Spatiotemporal distribution of seasonal bird assemblages on land-bridge islands: linking dynamic and static views of metacommunities. Avian Research, 10(1): 25. DOI: 10.1186/s40657-019-0164-7 | |
| Chuang Zhou, Shuai Zheng, Xue Jiang, Wei Liang, Megan Price, Zhenxin Fan, Yang Meng, Bisong Yue. 2018: First complete genome sequence in Arborophila and comparative genomics reveals the evolutionary adaptation of Hainan Partridge (Arborophila ardens). Avian Research, 9(1): 45. DOI: 10.1186/s40657-018-0136-3 | |
| Xiaodan Wang, Fenliang Kuang, Kun Tan, Zhijun Ma. 2018: Population trends, threats, and conservation recommendations for waterbirds in China. Avian Research, 9(1): 14. DOI: 10.1186/s40657-018-0106-9 | |
| Andrew J. Hamilton, Chloé Conort, Aurore Bueno, Christopher G. Murray, James R. Grove. 2017: Waterbird use of farm dams in south-eastern Australia: abundance and influence of biophysical and landscape characteristics. Avian Research, 8(1): 2. DOI: 10.1186/s40657-016-0058-x | |
| K. M. Aarif, Aymen Nefla, S. B. Muzaffar, K. K. Musammilu, P. K. Prasadan. 2017: Traditional fishing activities enhance the abundance of selected waterbird species in a wetland in India. Avian Research, 8(1): 16. DOI: 10.1186/s40657-017-0073-6 | |
| China Coastal Waterbird Census Group, Qingquan Bai, Jianzhong Chen, Zhihong Chen, Guotai Dong, Jiangtian Dong, Wenxiao Dong, Wing Kan Vivian Fu, Yongxiang Han, Gang Lu, Jing Li, Yang Liu, Zhi Lin, Derong Meng, Jonathan Martinez, Guanghui Ni, Kai Shan, Renjie Sun, Suixing Tian, Fengqin Wang, Zhiwei Xu, Yat-tung Yu, Jin Yang, Zhidong Yang, Lin Zhang, Ming Zhang, Xiangwu Zeng. 2015: Identification of coastal wetlands of international importance for waterbirds: a review of China Coastal Waterbird Surveys 2005-2013. Avian Research, 6(1): 12. DOI: 10.1186/s40657-015-0021-2 | |
| Zhifeng Ding, Kenneth James Feeley, Huijian Hu, Ping Ding. 2015: Bird guild loss and its determinants on subtropical land-bridge islands, China. Avian Research, 6(1): 10. DOI: 10.1186/s40657-015-0019-9 | |
| Wei LIANG, Zhengwang ZHANG. 2011: Hainan Peacock Pheasant (Polyplectron katsumatae): an endangered and rare tropical forest bird. Avian Research, 2(2): 111-116. DOI: 10.5122/cbirds.2011.0017 |
| Crossing behavior |
Flocks (a) | Solitary individuals (b) | |||
| EHB | WMH | EHB | WMH | ||
| Crossing | 128 (64%) | 87 (56%) | 107 (61%) | 88 (56.4%) | |
| Stopping | 72 (36%) | 68 (44%) | 69 (39%) | 68 (43.6%) | |
| Wind condition |
European Honey Buzzards | Western Marsh Harriers | |||||||||
| Flocks (a) | Solitary individuals (b) | Flocks (c) | Solitary individuals (d) | ||||||||
| Crossing | Stopping | Crossing | Stopping | Crossing | Stopping | Crossing | Stopping | ||||
| No wind | 34 (67%) | 17 (33%) | 33 (70%) | 14 (30%) | 19 (76%) | 6 (24%) | 41 (79%) | 11 (21%) | |||
| Head wind | 43 (58%) | 31 (42%) | 22 (42%) | 30 (58%) | 33 (44%) | 42 (56%) | 35 (57%) | 26 (43%) | |||
| Lateral wind | 15 (47%) | 17 (53%) | 15 (47%) | 17 (53%) | 23 (55%) | 19 (45%) | 14 (52%) | 13 (48%) | |||
| Time of the day |
European Honey Buzzards | Western Marsh Harriers | |||||||||
| Flocks (a) | Solitary individuals (b) | Flocks (c) | Solitary individuals (d) | ||||||||
| Crossing | Stopping | Crossing | Stopping | Crossing | Stopping | Crossing | Stopping | ||||
| Morning | 80 (77%) | 24 (23%) | 77 (74%) | 27 (26%) | 27 (96%) | 1 (4%) | 53 (91%) | 5 (9%) | |||
| Midday | 43 (61%) | 28 (39%) | 21 (46%) | 25 (54%) | 41 (59%) | 29 (41%) | 41 (57%) | 31 (43%) | |||
| Afternoon | 5 (20%) | 20 (80%) | 9 (35%) | 17 (65%) | 19 (33%) | 38 (67%) | 7 (27%) | 19 (73%) | |||
| Time of the day | No wind | Head wind | Lateral wind |
| Morning | 40 (61%) | 9 (14%) | 16 (25%) |
| Midday | 20 (19%) | 59 (55%) | 28 (26%) |
| Afternoon | 15 (14%) | 58 (55%) | 32 (31%) |
| Total | 75 (27%) | 126 (46%) | 76 (27%) |
| Age class | European Honey Buzzards (a) | Western Marsh Harriers (b) | |||||
| Crossing | Stopping | Flying along the coast |
Crossing | Stopping | Flying along the coast |
||
| Adults | 100 (59%) | 58 (34%) | 11 (7%) | 148 (48%) | 154 (50%) | 7 (2%) | |
| Juveniles | 238 (55%) | 157 (37%) | 34 (8%) | 66 (59%) | 43 (38%) | 3 (3%) | |