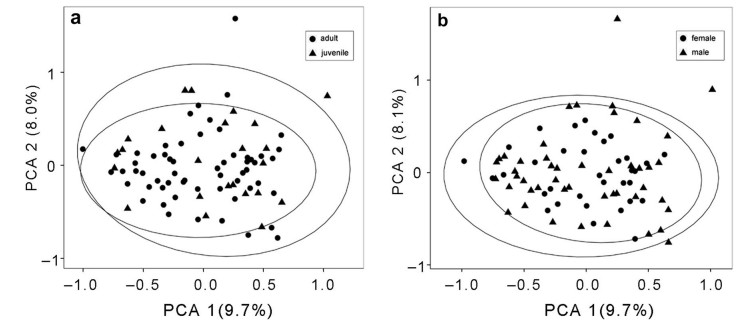
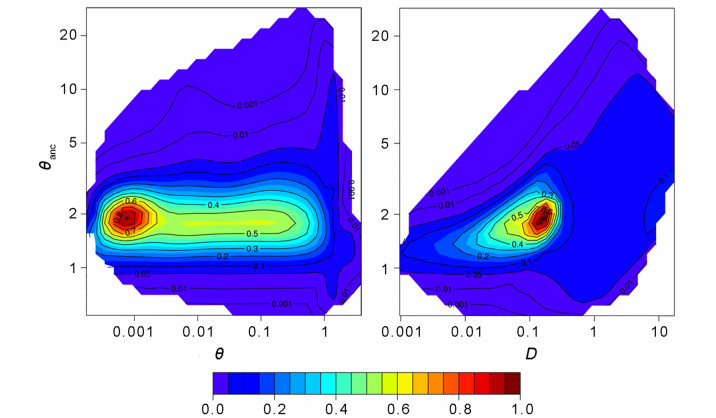
|
Allendorf FW, Luikart G. Conservation and the genetics of populations. New York: Wiley; 2009.
|
|
Amos W, Balmford A. When does conservation genetics matter? Heredity. 2001;87:257-65.
|
|
Baker RR. The evolutionary ecology of animal migration. New York: Holmes & Meier Publishers; 1978.
|
|
Belkhir K. GENETIX, logiciel sous WindowsTM pour la génétique des populations. 1999. . Accessed 24 Mar 2018.
|
|
BirdLife International. Birds in Europe: population estimates, trends and conservation status. Cambridge: BirdLife Conservation Series; 2004.
|
|
Catsadorakis G. The importance of Prespa National Park for breeding and wintering birds. Hydrobiologia. 1997;351:157-74.
|
|
Catsadorakis G, Voslamber B, Logotheti A. First Greylag Geese Anser anser rubrirostris ringed in Greece. Goose Bull. 2012;15:28-31.
|
|
Chapman J, Nakagawa S, Coltman D, Slate J, Sheldon B. A quantitative review of heterozygosity-fitness correlations in animal populations. Mol Ecol. 2009;18:2746-65.
|
|
Coltman D, Pilkington J, Smith J, Pemberton J. Parasite mediated selection against inbred Soay sheep in a free-living island population. Evolution. 1999;53:1259-67.PubMed
|
|
Cornuet JM, Luikart G. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics. 1996;144:2001-14.
|
|
Cristescu R, Sherwin WB, Handasyde K, Cahill V, Cooper DW. Detecting bottlenecks using BOTTLENECK 1.2. 02 in wild populations: the importance of the microsatellite structure. Conserv Genet. 2010;11:1043-9.
|
|
Crnokrak P, Roff DA. Inbreeding depression in the wild. Heredity. 1999;83:260-70.
|
|
David P, Pujol B, Viard F, Castella V, Goudet J. Reliable selfing rate estimates from imperfect population genetic data. Mol Ecol. 2007;16:2474-87.
|
|
Di Rienzo A, Peterson AC, Garza JC, Valdes AM, Slatkin M, Freimer NB. Mutational processes of simple sequence repeat loci in human populations. Proc Natl Acad Sci USA. 1994;91:3166-70.
|
|
Do C, Waples RS, Peel D, Macbeth G, Tillett BJ, Ovenden JR. NeEstimator v2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol Ecol Res. 2014;14:209-14.
|
|
Excoffier L, Lischer HE. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res. 2010;10:564-7.
|
|
Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567-87.
|
|
Forstmeier W, Schielzeth H, Mueller JC, Ellegren H, Kempenaers B. Heterozygosity-fitness correlations in zebra finches: microsatellite markers can be better than their reputation. Mol Ecol. 2012;21:3237-49.
|
|
Fox AD, Ebbinge BS, Mitchell C, Heinicke T, Aarvak T, Colhoun K, Clausen P, Dereliev S, Faragó S, Koffijberg K. Current estimates of goose population sizes in western Europe, a gap analysis and assessment of trends. Ornis Svec. 2010;20:115-27.
|
|
Frankham R. Effective population size/adult population size ratios in wildlife: a review. Genet Res. 1995;66:95-107.
|
|
Frankham R, Briscoe DA, Ballou JD. Introduction to conservation genetics. Cambridge: Cambridge University Press; 2002.
|
|
Freeland JR. Molecular ecology. New York: Wiley; 2005.
|
|
Freeman S, Jackson WM. Univariate metrics are not adequate to measure avian body size. Auk. 1990;107:69-74.
|
|
Friesen V, Burg T, McCoy K. Mechanisms of population differentiation in seabirds. Mol Ecol. 2007;16:1765-85.
|
|
Garza JC, Williamson EG. Detection of reduction in population size using data from microsatellite loci. Mol Ecol. 2001;10:305-18.
|
|
Gosler A. Pattern and process in the bill morphology of the Great Tit Parus major. Ibis. 1987;129:451-76.
|
|
Goudet J. FSTAT, a program to estimate and test gene diversities and fixation indices version 2.9.3.2. 2002. . Accessed 20 Aug 2012.
|
|
Grueber CE, Wallis GP, Jamieson IG. Heterozygosity-fitness correlations and their relevance to studies on inbreeding depression in threatened species. Mol Ecol. 2008;17:3978-84.
|
|
Harrison XA, Bearhop S, Inger R, Colhoun K, Gudmundsson GA, Hodgson D, McElwaine G, Tregenza T. Heterozygosity-fitness correlations in a migratory bird: an analysis of inbreeding and single-locus effects. Mol Ecol. 2011;20:4786-95.
|
|
Hedrick PW, Kalinowski ST. Inbreeding depression in conservation biology. Annu Rev Ecol Evol Syst. 2000;31:139-62.
|
|
Heikkinen M, Ruokonen M, Alexander M, Aspi J, Pyhäjärvi T, Searle J. Relationship between wild greylag and European domestic geese based on mitochondrial DNA. Anim Genet. 2015;46:485-97.
|
|
Hewitt GM. Post-glacial re-colonization of European biota. Biol J Linn Soc. 1999;68:87-112.
|
|
Hill WG. Estimation of effective population size from data on linkage disequilibrium. Genet Res. 1981;38:209-16.
|
|
Höglund J, Piertney SB, Alatalo RV, Lindell J, Lundberg A, Rintamäki PT. Inbreeding depression and male fitness in black grouse. Proc R Soc B. 2002;269:711-5.
|
|
Horvath MB, Martinez-Cruz B, Negro JJ, Kalmar L, Godoy JA. An overlooked DNA source for non-invasive genetic analysis in birds. J Avian Biol. 2005;36:84-8.
|
|
Jonker R, Kraus RH, Zhang Q, Hooft P, Larsson K, Jeugd H, Kurvers R, Wieren S, Loonen M, Crooijmans R. Genetic consequences of breaking migratory traditions in barnacle geese Branta leucopsis. Mol Ecol. 2013;22:5835-47.
|
|
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870-4.
|
|
Lagnel J. ParaStructure. 2015. . Accessed 22 Mar 2018.
|
|
Leblois R, Pudlo P, Néron J, Bertaux F, Reddy Beeravolu C, Vitalis R, Rousset F. Maximum-likelihood inference of population size contractions from microsatellite data. Mol Biol Evol. 2014;31:2805-23.
|
|
Møller AP, Fiedler W, Berthold P. Effects of climate change on birds. Oxford: Oxford University Press; 2010.
|
|
Moritz C. Defining 'evolutionarily significant units' for conservation. Trends Ecol Evol. 1994;9:373-5.
|
|
Navidi W, Arnheim N, Waterman M. A multiple-tubes approach for accurate genotyping of very small DNA samples by using PCR: statistical considerations. Am J Hum Genet. 1992;50:347.
|
|
Nei M, Tajima F. Genetic drift and estimation of effective population size. Genetics. 1981;98:625-40.
|
|
Newton I. The migration ecology of birds. London: Academic Press; 2010.
|
|
Palacín C, Alonso JC, Martín CA, Alonso JA. Changes in bird-migration patterns associated with human-induced mortality. Conserv Biol. 2017;31:106-15.
|
|
Palsbøll PJ, Berube M, Allendorf FW. Identification of management units using population genetic data. Trends Ecol Evol. 2007;22:11-6.
|
|
Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics. 2012;28:2537-9.
|
|
Pearce JM, Talbot SL, Pierson BJ, Petersen MR, Scribner KT, Dickson DL, Mosbech A. Lack of spatial genetic structure among nesting and wintering King Eiders. Condor. 2004;106:229-40.
|
|
Pellegrino I, Cucco M, Follestad A, Boos M. Lack of genetic structure in greylag goose (Anser anser) populations along the European Atlantic flyway. PeerJ. 2015;3:e1161.
|
|
Piry S, Luikart G, Cornuet J-M. Computer note. BOTTLENECK: a computer program for detecting recent reductions in the effective size using allele frequency data. J Hered. 1999;90:502-3.
|
|
Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945-59.
|
|
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2017.
|
|
Rohwer FC, Anderson MG. Female-biased philopatry, monogamy, and the timing of pair formation in migratory waterfowl. In: Johnston E, Richard F, editors. Current ornithology. Boston: Springer; 1988. p. 187-221.
|
|
Ruokonen M, Kvist L, Lumme J. Close relatedness between mitochondrial DNA from seven Anser goose species. J Evol Biol. 2000;13:532-40.
|
|
Scott DA, Rose PM. Atlas of Anatidae populations in Africa and western Eurasia. Ede: Wetlands International; 1996.
|
|
Slate J, David P, Dodds K, Veenvliet B, Glass B, Broad T, McEwan J. Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: theoretical expectations and empirical data. Heredity. 2004;93:255-65.
|
|
Stoffel MA, Esser M, Kardos M, Humble E, Nichols H, David P, Hoffman JI. InbreedR: an R package for the analysis of inbreeding based on genetic markers. Methods Ecol Evol. 2016;7:1331-9.
|
|
Svensson L. Identification guide to European passerines. Thetford: British Trust for Ornithology; 1992.
|
|
Szulkin M, Bierne N, David P. Heterozygosity-fitness correlations: a time for reappraisal. Evolution. 2010;64:1202-17.PubMed
|
|
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673-80.
|
|
Toonen RJ, Hughes S. Increased throughput for fragment analysis on an ABI Prism® 377 automated sequencer using a membrane comb and STRand software. Biotechniques. 2001;31:1320-5.PubMed
|
|
Valière N. GIMLET: a computer program for analysing genetic individual identification data. Mol Ecol Notes. 2002;2:377-9.
|
|
van der Jeugd HP, van der Veen IT, Larsson K. Kin clustering in barnacle geese: familiarity or phenotype matching? Behav Ecol. 2002;13:786-90.
|
|
Van Oosterhout C, Hutchinson WF, Wills DP, Shipley P. MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes. 2004;4:535-8.
|
|
Waples RS. A bias correction for estimates of effective population size based on linkage disequilibrium at unlinked gene loci. Conserv Genet. 2006;7:167.
|
|
Weiß BM, Poggemann K, Olek K, Foerster K, Hirschenhauser K. Isolation and characterization of microsatellite marker loci in the greylag goose (Anser anser). Mol Ecol Res. 2008;8:1411-3.
|
|
Wickham H, Chang W. Ggplot2: an implementation of the grammar of graphics. 2015. . Accessed 30 Mar 2018.
|
|
Willoughby JR, Sundaram M, Wijayawardena BK, Lamb MC, Kimble SJ, Ji Y, Fernandez NB, Antonides JD, Marra NJ, Dewoody JA. Biome and migratory behaviour significantly influence vertebrate genetic diversity. Biol J Linn Soc. 2017;121:446-57.
|
 必应学术
必应学术
 必应学术
必应学术
 必应学术
必应学术
 必应学术
必应学术


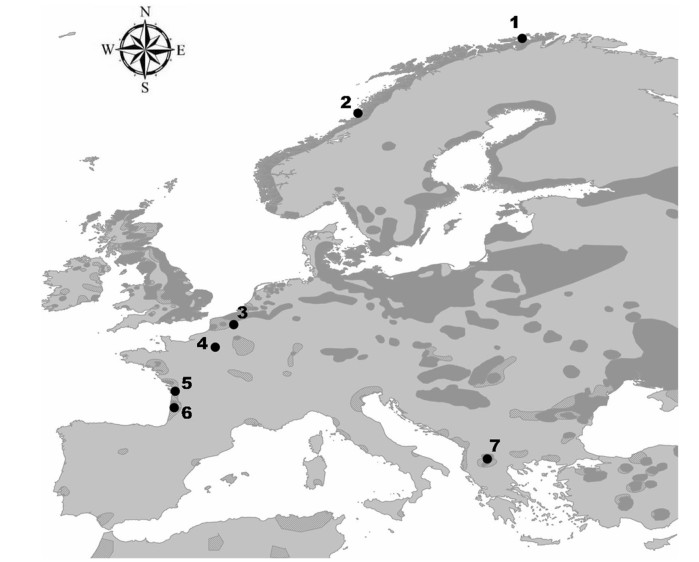
 下载:
下载: