Birds of the Kangchenjunga Landscape, the Eastern Himalaya: status, threats and implications for conservation
-
Abstract:
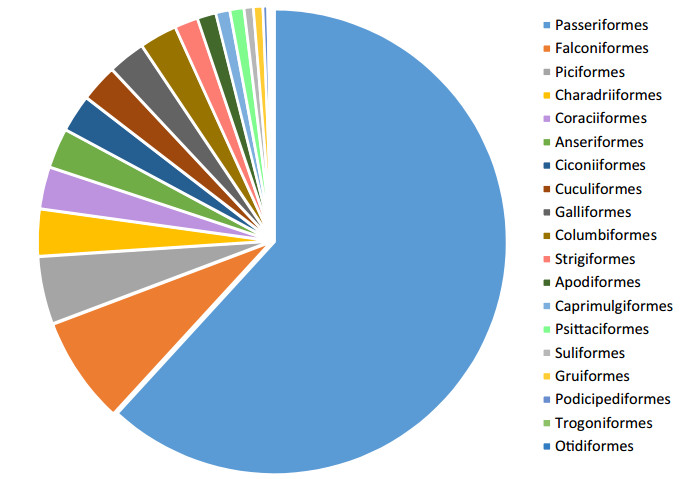
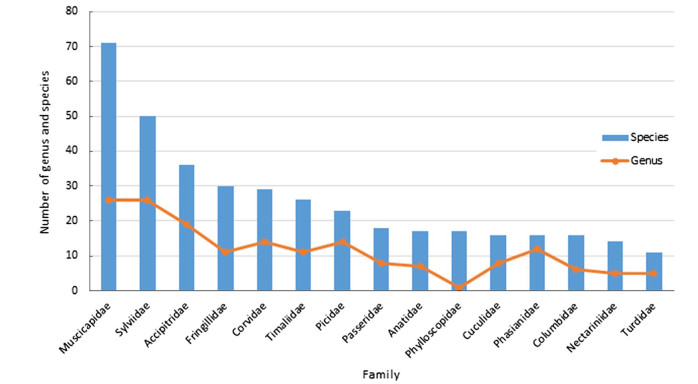
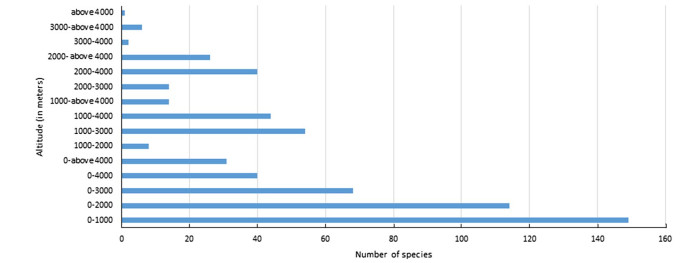
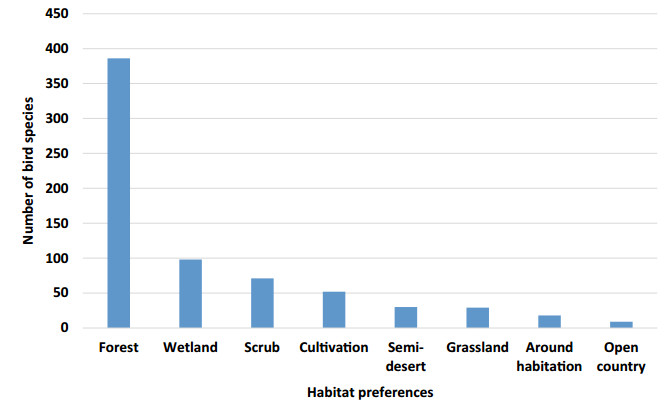
Birds are reliable and widely used indicators for conservation planning and monitoring. We reviewed birds of the Kangchenjunga Landscape, a transboundary complex shared by Bhutan, India and Nepal in the Eastern Himalaya. Using 119 literature, we analyzed the bird survey efforts in the landscape, their taxonomic representation, global threat status, distribution patterns, and habitat preferences. We also discussed the potential threats and conservation challenges and documented current conservation efforts and government policies. Most of the bird surveys are carried out in India followed by Nepal and Bhutan. A total of 618 bird species belonging to 19 orders and 77 families are recorded. Passeriformes is the dominant order that constitutes 62% of the total records listed from the landscape. Among the families, Muscicapidae is the most common and diversely represented family. There are 41 species of birds that are categorized as threatened under IUCN Red List. Of the total birds occurring in the landscape, the highest number of bird species (95%) was documented from India, followed by Nepal (55%) and Bhutan (34%). Of them, 24% of the species were found to occur in the tropical zone. Forested habitat is widely used by 63% of the total species followed by wetlands (16%). Despite promising policies and legal provisions, the landscape faces numerous challenges including habitat loss and fragmentation, hunting and trapping, unsustainable extraction of natural resources, invasive alien species, unregulated tourism and global climate change. We suggest protection and management of birds through strengthening Important Bird and Biodiversity Areas, reduction in forest encroachment and habitat destruction, conservation awareness programmes and comprehensive bird surveys with long term monitoring to assess the impact of environmental change as some of the approaches to conserve the rich avifaunal diversity of the landscape.
-
Keywords:
- Avifauna /
- Transboundary landscape /
- Nepal /
- India /
- Bhutan /
- Biodiversity /
- Conservation
-
Background
In altricial birds, parenting behaviors are critical for the survival of dependent offspring (Silver et al. 1985; Tallamy and Wood 1986) while they are costly for parents due to their time- and energy-consuming (Skutch 1949). It has long been suggested by the life-history theory that parents should optimize their parenting patterns under a specific environmental condition in a way that would maximize their reproductive fitness (Lack 1954). Empirical tests of the life-history theory have revealed an astonishing diversity in altricial birds' parenting care patterns (Winkler and Wallin 1987; Martin 1995; Sæther and Bakke 2000; Eikenaar et al. 2003; Martin et al. 2011; Du et al. 2014). These patterns are generally considered as an adaptive response to various environmental conditions, such as food availability and nest predation risk (Martin 1995; Caro et al. 2016). However, few studies have attached importance to the differentiation in life-history style in driving avian parenting behaviors to diversify.
One fundamental characteristic of life-history style is the time that an individual can breed a year. Most passerine species, only when the length of breeding season supports multiple broods could an individual select to breed one or more times (Verhulst and Nilsson 2008; Camfield et al. 2010; Du et al. 2014; Li et al. 2020a). Single- and multi-brooded breeders exhibit different tradeoffs in their parenting patterns. Multi-brooded individuals often adopt different strategies across broods. It will be beneficial for breeders to adopt a brood reduction strategy when the breeding conditions are poor, i.e. biasing investment towards stronger offspring, to ensure the lowest nest survival threshold (Mock and Forbes 1994; Forbes et al. 2001). When breeding conditions are better, it will be beneficial for breeders to adopt a brood survival strategy, i.e. biasing investment towards weaker offspring, so that they can raise many offspring as possible (Slagsvold et al. 1984, 1997; Forbes 2007). In contrast, single-brood species are more likely to adopt the brood survival strategy regardless of the breeding conditions, as they have only one chance to realize their fitness (Du et al. 2012; Li et al. 2020a). A comparison between sympatric single- and multi-brooded species' parenting strategies would help understand the role of life-history style in the diversity of avian parenting behaviors.
Altricial birds have two important breeding phases, the egg and the nestling, to optimize their parenting strategies. In the egg phase, parents trade off the eggs' number and size mainly based on the expected amount of available food (egg size strategy; Slagsvold et al. 1984; Christians 2002); whereas, they select their brood provisioning strategy based on the actual amount of food in the nestling phase (Decker et al. 2012). By modulating an egg's size according to its laying sequence, female birds can affect the size hierarchy among newly-hatched offspring, which in turn affects the intensity of subsequent sibling rivalry (Shizuka and Lyon 2013; Mainwaring et al. 2014). When the egg size decreases with the laying sequence, as in the Giant Babax (Babax wadelli), the last hatchling is smaller than its older siblings and hence at a disadvantage in the competition over parental investment within the brood (Du et al. 2012). In contrast, when the egg size increases with the laying sequence, as in the Azure-winged Magpie (Cyanopica cyanus), later hatchlings are larger at hatching than their older brood-mates. This can compensate, to some extent, for the disadvantage faced by later offspring in competing with their older siblings (Da et al. 2018). In the tradeoff between nestlings' number and size, parents in many cases adopt different parenting strategies from that in egg-laying, particularly in an environment where the breeding conditions are unpredictable (Decker et al. 2012). For example, parents of the Giant Babax adopt the "brood reduction" strategy in laying eggs and the "brood survival" strategy in provisioning the nestlings (Du et al. 2012); while parents of the Horned Lark (Eremophila alpestris) adopt the "brood survival" strategy in laying eggs and the "brood reduction" strategy in provisioning the nestlings (Du et al. 2014; Da et al. 2018). The difference in individual tradeoffs between egg-laying and nestling-provisioning has become a common explanation for parenting pattern evolution, whereas the effect of life-history style has been largely neglected.
In this study, we addressed the role of life-history style in the evolution of parenting patterns in the Grey-backed Shrike (Lanius tephronotus), which is a small (approximately 40 g), carnivorous bird with no sexual dimorphism neither in size nor in plumage. It is the only Lanius species that can breed at the high elevation of the Tibetan Plateau (ranging between 2700 and 4500 m) (Lu et al. 2010). Generally, Grey-backed Shrikes produce only one brood a year. In a population distributed in their upper range limit (4000–4500 m), the clutch size (ranging from 3 to 5) decreases significantly with the elevation (Lu et al. 2010). In contrast, in the population distributed in the lower range limit on the Tibetan Plateau (2600–2900 m), females tend to produce a fixed clutch size of five (B. Du, unpublished data). It seems that Grey-backed Shrikes have made adaptive responses to the variation of breeding conditions; hence, it might be an ideal system to compare individual tradeoffs between egg-laying and nestling-provisioning. Several shrub-nesting bird species, such as the White-collared Blackbirds (Turdus albocinctus) (Fan et al. 2017) and the Brown-cheeked Laughing Thrush (Trochalopteron henrici) (Li et al. 2020b), are sympatric in the Grey-backed Shrike's lower range limit. Both species mix with the Grey-backed Shrike in their nesting and foraging sites, but can breed twice a year. Therefore, it becomes possible to compare the parenting strategy of sympatric species, to identify the relative role of life-history style and breeding conditions in driving parenting behavior evolution.
To address whether Grey-backed Shrikes adopt different strategies between the egg and nestling phases, we first identified their egg-laying pattern and the growth pattern of nestling body mass with different hatching sequences. Then, we also performed a dietary investigation and food types between the Grey-backed Shrike and Brown-cheeked Laughing Thrush to examine the life-history style's effect on the differentiation of parenting patterns.
Methods
Study area and population
This study was carried out in the Bayi town, Tibetan Autonomous Region, China (29°40′N, 94°20′E, mean altitude of 2900 m), during 2015–2019. This region has a typical cold (mean annual temperature 7℃) and wet (annual precipitation 500–700 mm) high-altitude climate. The temperature and precipitation change greatly through the year, with the highest temperatures and rainfall occurring between June and August (Fan et al. 2017). Local landscape is characterized by the evergreen Chuan-Dian Alpine Oak (Quercus aquifolioides) forest, mixed with some deciduous trees, such as the Aspen (Populus davidiana) and Tibetan Willow (Salix insignis), and shrubs composed mainly of the Lhasa Berberis (Berberis hemsleyana), roses (Rose spp.), azaleas (Rhododendron spp.), and powder-branched berry (Rubus biflorus). Our study area of 300 ha is located along the Niyang River, where Grey-backed Shrikes choose to build most of their nests in the shrubs.
Grey-backed Shrikes breed once a year in our study area. At the end of June, pairs start occupying territories and build their nests in the shrubs. Nest construction is carried out mainly by the female, while the male defends the territory. The outmost layer of a nest comprises small branches, withered grass, and plastic sheeting pieces, while the inner layer is lined with fine grass stems and animal hair. Females lay their eggs immediately after the nest is constructed. After the first egg is laid, the female would brood the nest, but only at night; after the penultimate egg was laid, all-day incubation is commenced. After that, both sexes contribute to the incubation. Hatching asynchrony occurs in the Grey-backed Shrike, with three or four chicks hatching in the first day and the remaining on the second day. Both sexes contribute to provisioning nestlings during the nestling period and approximately one month after they fledge.
Data collection for reproductive parameters
Data collection for reproductive parameters began with a systematic search for the Grey-backed Shrike nests at the end of June. The nest contents were checked daily to determine the clutch initiation date (the date when the first egg was laid) and the laying sequence of each egg. The laying sequence was marked on its eggshells with a non-toxic marker pen (Deli Company, Guangzhou, Guangdong Province, China). The fresh mass as an index for its size, was measured with an electronic balance to the nearest 0.1 g. The hatching sequence was marked on the chicks' heads at hatching, and their body mass was measured (to the nearest 0.1 g). When two or more nestlings hatched on the first day, their hatching sequences could be determined according to their skin color. The darker the color, the earlier a nestling hatched. During the nestling period, nest content was checked every two days to measure the nestlings' body mass. When the nestlings reached 30 g or were older than ten days, they were leg-banded with one numbered metal ring and two colored plastic rings. Nesting success was considered achieved when a social pair fledged at least one offspring.
Adults were captured after the nestlings have hatched, using a method that had been successfully adopted to capture other shrub-nesting species, such as the Azure-winged Magpie (Ren et al. 2016) and the White-collared Blackbird (Fan et al. 2017). Capturing the adults and measuring the nestlings were performed under the permission of the Tibetan Forestry Department (2016ZR-NY-05). Each captured individual was sexed by the presence of a brooding patch, leg-banded with one numbered aluminum ring and two colored plastic rings, and weighed. Only one parent was captured in most nests to minimize the disturbance on parenting behaviors. This procedure fulfilled the parents' sex identification requirement.
A dietary investigation was performed during the nestling period to examine the type and size of food that Grey-backed Shrikes delivered to their offspring. First, adults foraging behaviors were monitored to determine their foraging sites. Then, different types of food were sampled at the foraging sites by searching for insects on the ground and in the earth, and by gathering fruits berries as, in some cases, adults were found to feed on such plant food. The different types of food were weighed and classified based on their mean mass (Additional file 1: Table S1). These were considered candidate dietary items that parents might deliver to their offspring.
Data collection for parental provisioning behaviors
After adults were leg-banded, their provisioning behaviors were recorded automatically by digital camcorders (ZX1, Eastman Kodak Company, Rochester, NY, USA) for 3 h (9:00–12:00 a.m., China Standard Time) every two days. Each camcorder was mounted on a tripod that was fixed diagonally 0.8–1 m above a nest. The recording process caused the Grey-backed Shrike no adverse effects as there were no nest abandonment cases during the recording periods. A total of 256 h of adult provisioning behavior recordings were obtained (13.5 ± 0.7 h per nest, n = 19 nests).
Data on parental provisioning behaviors were extracted from videos by playing them back on a computer. This dataset included: (1) identity of the nest-visitor and whether it delivered food to the brood; (2) the type, number, size of food a provisioner delivered to the nestlings; (3) the predators' species that were monitored preying on eggs or nestlings. Based on these data, an individual's provisioning rate was calculated as the number of feeding bouts per hour; the food types and sizes identified in the video were assigned to the candidate food list (Additional file1: Table S1), so that the amount of food a parent delivered to the brood per feeding bout could be calculated.
Statistical analysis
A generalized linear mixed model (GLMM) was fitted to test factors that might influence the fresh egg mass set as a dependent variable, with identical link function (Table 1). Fixed factors included the clutch initiation date and the egg laying sequence. The clutch size was not included in the model because it seemed stable among the nests. Random effects included the year and nest identity. A GLMM was also fitted to test factors that might influence the nestlings' body mass set as a dependent variable, with identical link function (Table 2). Fixed factors included the clutch initiation date, nestling age, the hatching sequence. Random effects included the year, nest identity, and nestling identity. Factors that might influence the provisioning rate of males and females set as a dependent variable, with identical link function (Table 3), were tested by fitting two more GLMMs. Fixed effects included brood size and nestling age, and random effects included the year and nest identity. Similarly, factors that might influence the food amount delivered by males or females per feeding bout (set as dependent variable), with identical link function (Table 4) were tested by fitting GLMMs. Fixed effects included brood size, nestling age, the breeder's provisioning rate, and random effects included the year and nest identity. During the process of fitting GLMMs, we did not introduce parental body conditions into the model because parents were captured in different nestling ages, and in most nests, only one parent was captured. As a substitute, we performed variance component analysis (VCA) to examine the relative contribution of between-nest difference (i.e. the random effect of nest identity) to the variance in each dependent variable. Multiple linear regression was used to test the multicollinearity of fixed effect variables before fitting the GLMMs. Variables were considered to have serious multicollinearity when their variance inflation factor (VIF) was larger than three.
Table 1. Factors that might influence the egg's fresh mass in the Grey-backed ShrikeGeneralized linear mixed model parameters Fixed effects β ± SE n t P Intercept 4.274 ± 0.094 151 45.618 < 0.001 Clutch initiation date – 0.002 ± 0.003 151 – 0.555 0.580 Laying sequence 0.058 ± 0.016 151 3.697 < 0.001 Random effects β ± SD n Results of VCA (%) Nest identity 0.097 ± 0.311 151 66.90 Years 0.001 ± 0.001 151 0.69 Residual 0.047 ± 0.217 151 32.41 SE of fixed effects is the standard error of the mean; SD of random effects is the square root of the variance. The explanations apply also to Table 2, 3 and 4 Table 2. Factors that might influence the nestling's body mass in the Grey-backed ShrikeGeneralized linear mixed model parameters Fixed effects β ± SE n t P Intercept 1.620 ± 1.086 284 1.492 0.065 Clutch initiation date 0.042 ± 0.048 284 0.870 0.284 Hatching sequence – 0.186 ± 0.164 284 – 1.134 0.101 Nestling age 2.264 ± 0.054 284 42.234 < 0.001 Random effects β ± SD n Results of VCA (%) Nestling identity 0.001 ± 0.001 284 0.006 Nest identity 3.694 ± 1.922 284 21.25 Year 3.032 ± 1.741 284 17.44 Residual 10.659 ± 3.265 284 61.31 Table 3. Factors that might influence the provisioning rate of males and females in the Grey-backed ShrikeParameters
Fixed effectsMales Females β ± SE t n P β ± SE t n P Intercept 2.38 ± 5.55 0.43 143 0.67 – 8.55 ± 7.55 – 1.13 143 0.26 Brood size 1.10 ± 0.80 1.38 143 0.17 3.20 ± 1.05 3.04 143 0.003 Nestling age 0.33 ± 0.10 3.15 143 0.002 0.81 ± 0.17 4.70 143 < 0.001 Random effects β ± SD Results of VCA (%) β ± SD Results of VCA (%) Year 0.001 ± 0.001 0.001 6.40 ± 2.53 11.39 Nest identity 0.02 ± 0.15 0.07 0.001 ± 0.001 0.001 Residual 30.16 ± 5.49 99.92 49.75 ± 7.05 88.61 Table 4. Factors that might influence the food amount delivered by males or females per feeding bout in the Grey-backed ShrikeParameters
Fixed effectsMale Female β ± SE t n P β ± SE t n P Intercept 0.74 ± 0.83 0.89 1470 0.37 1.00 ± 0.76 1.31 1817 0.19 Brood size 0.36 ± 0.06 6.03 1470 < 0.001 0.21 ± 0.06 3.50 1817 < 0.001 Nestling age 0.01 ± 0.01 0.75 1470 0.46 0.27 ± 0.01 2.76 1817 0.006 Provisioning rate – 0.02 ± 0.01 – 4.08 1470 < 0.001 – 0.02 ± 0.03 – 9.14 1817 < 0.001 Random effects β ± SD Results of VCA (%) β ± SD Results of VCA (%) Year 0.02 ± 0.14 2.17 0.04 ± 0.19 5.01 Nest identity 0.14 ± 0.37 14.30 0.03 ± 0.17 4.06 Residual 0.80 ± 0.89 83.53 0.68 ± 0.82 90.93 A logistic model was fitted to monitor the nestlings' growth pattern based on their body mass, using non-linear regression (Huin and Prince 2000): W = K / (1 + exp(a – b × A)). In this equation, W is the nestling's body mass, K is the asymptotic body mass that a fledgling could reach, a is the nestling's exponential growth initiation date, b is the instantaneous growth rate, and A is the nestling age. The fledgling body mass of nestlings was compared by one-way ANOVA based on their hatching sequence.
The different food types frequencies delivered by parents to their offspring were tested to examine whether they were distributed evenly, using the one sample Kolmogorov–Smirnov test.
All analyses were conducted using SPSS (version 21.0; IBM Corp, Armonk, NY, USA) and R (version 3.3.4). Descriptive results are presented as mean ± standard error (SE). The null hypothesis was rejected when P < 0.05, and reported probabilities are two-tailed.
Results
Over four years (2015–2018), 59 Grey-backed Shrikes nests were observed to complete their clutches in our study area. Females tended to produce a fixed clutch size of five (97% of the nests, 57/59). The mean fresh egg mass was 4.5 ± 0.4 g (n = 151, range 3.6–5.7 g). Thirty-one nests fledged at least one offspring, with a mean brood size of 4.0 ± 0.2 (n = 31 broods). The Domestic Cat (Felis catus) that preyed on the eggs and nestlings was found to be the main predator of the Grey-backed Shrike.
Variation of egg size with their laying sequence
The fresh egg mass did not vary with the clutch initiation date, but it changed significantly with the laying sequence (Table 1). The later an egg was laid, the larger it was (F4, 146 = 2.52, P = 0.04; Fig. 1). The variance of eggs' fresh mass was greater between nests than between years (Table 1).
Growth pattern in nestlings' body mass
A nestling's body mass increased significantly with age but did not differ between clutch initiation date or its hatching sequence (Table 2). The variance of nestling's body mass was greater between nests or years than between nestlings (Table 2).
The last nestlings in a brood were the biggest offspring, whereas the penultimate nestlings were the smallest ones (Fig. 2). As the last nestlings were usually one day younger than their brood-mates, the body mass growth patterns differed significantly among brood-mates (Fig. 2).
Provisioning patterns of the Grey-backed Shrike
The provisioning rate increased significantly with the nestling age in both males and females, but increased with brood size only in females (Table 3). Males' provisioning rate variance was greater between nests than between years; whole for females, it was greater between years than between nests (Table 3).
Food amount delivered by males per feeding bout increased with the brood size but decreased with the provisioning rate in both males and females. It changed with the nestling age only in females (Table 4). The variance of males' food amount per feeding bout is greater between nests than between years, while it was the same between nests and between years in females (Table 4).
Males' provisioning rate (8.92 ± 0.58 bouts/h, n = 16 days) was significantly lower than that of females (11.17 ± 1.09 bouts/h, n = 16 days; t = – 2.24, df = 15, P = 0.04; Fig. 3a). However, the amount of food males delivered per feeding bout (0.17 ± 0.003 bouts/h, n = 16 days) was significantly higher than that of females (0.15 ± 0.003 bouts/h, n = 16 days; t = 5.84, df = 15, P = 0.001; Fig. 3b). As a result, males' contribution to provisioning of offspring (49.06 ± 2.86%, n = 16 days) was the same as that of females (50.94 ± 2.86%, n = 16 days; t = 0.33, df = 15, P = 0.75).
Dietary composition of the Grey-backed Shrike
The dietary investigation identified eleven types of food that parents had delivered to their offspring (Fig. 4). The frequencies of the different food types are unevenly distributed (Z = 1.48, n = 11, P = 0.03). Lepidoptera larva and Hymenoptera adult were the two main components, contributing 29.07% and 24.27% to the diet, respectively; plant food, mainly powder-branched berry, was the least prevalent component, contributing only 0.15% to the diet (Fig. 4).
![Figure 4. Frequency of different food types that parents deliver to their offspring in the Grey-backed Shrikes (black column), as well as the Brown-cheeked Laughing Thrushes in their first (blank columns) and second breeding attempt (red columns). Data supporting this result are provided in Additional file 1: Table S2]() Figure 4. Frequency of different food types that parents deliver to their offspring in the Grey-backed Shrikes (black column), as well as the Brown-cheeked Laughing Thrushes in their first (blank columns) and second breeding attempt (red columns). Data supporting this result are provided in Additional file 1: Table S2
Figure 4. Frequency of different food types that parents deliver to their offspring in the Grey-backed Shrikes (black column), as well as the Brown-cheeked Laughing Thrushes in their first (blank columns) and second breeding attempt (red columns). Data supporting this result are provided in Additional file 1: Table S2The dietary items of the Grey-backed Shrike exhibit two major differences compared with the Brown-cheeked Laughing Thrush. First, nearly one quarter of the Grey-backed Shrike's diet is adult Hymenoptera that was not listed as food for Brown-cheeked Laughing Thrush. Yet, more than a quarter of the Brown-cheeked Laughing Thrush's diet is adult Diptera, followed by powder-branched berry (Fig. 4). Second, Grey-backed Shrikes rely mainly on animal food, whereas Brown-cheeked Laughing Thrushes mix animal and plant food to raise their offspring (Fig. 4). Moreover, meat has contributed 5.12% to the Grey-backed Shrike diet (Fig. 4). We recorded one case in which parents tore down a chick that had died in the nest and fed it to the remaining nestlings.
Discussion
Grey-backed Shrike parents adopt a brood survival strategy in both the egg and nestling phases. Such a strategy is not only an adaptive response to the local environmental conditions, but also a consequence of the Grey-backed Shrike life-history style of one brood a year and a wide range of dietary items.
Brood survival strategy of the Grey-backed Shrike in egg-laying
In our study area, female Gray-backed Shrikes' egg-laying strategy differs from the similar egg size strategy found in altricial birds, often manifested by a tradeoff between the number and size of eggs (Slagsvold et al. 1984; Martin 1987). Under preferable environmental conditions, such as lower nest predation risk and plentiful food supply, parents tend to lay larger clutches of smaller eggs; whereas under poor environmental conditions, such as higher nest predation risk and food scarcity, they are more likely to lay smaller clutches of larger eggs (Forbes 1993; Forbes et al. 2001; Du et al. 2012). Grey-backed Shrikes in our study area (i.e. the lower limit of their distribution on the Tibetan Plateau) produce clutches with a fixed size, unlike the population breeding in the upper limit of their Tibetan Plateau distribution. The population produces unstable clutches of decreasing size with altitude (Lu et al. 2010). Under such conditions, females only need to modulate the egg size based on the laying sequence. It would be a simpler strategy than trading off between the number and size of eggs in coping with the local environmental conditions. As the egg size increases with the laying sequence (Table 1), female Grey-backed Shrikes seem to adopt the brood survival strategy by modulating the egg size. This modulation facilitates parents to compensate for the disadvantage faced by the later offspring. After all, the greater the investment parents put into their later eggs, the more likely those offspring are to survive (Du et al. 2014; Da et al. 2018).
Brood survival strategy of the Grey-backed Shrike in brood provisioning
During the nestling period, the last offspring had higher growth rate than their brood-mates, implying that Grey-backed Shrike parents also adopt the brood survival strategy in brood provisioning. In many altricial birds, such as the Horned Larks (Du et al. 2014) and Black-collared Blackbirds (Fan et al. 2017), parents adopt the brood reduction strategy in provisioning their nestlings while they adopt the brood survival strategy when laying their eggs. This difference in parenting strategies between the egg and nestling phases may have resulted from a tradeoff between multiple yearly breeding cycles (Fan et al. 2017; Li et al. 2020a) or between the current and future reproduction (Trivers 1972; Palmer et al. 2004). In both tradeoffs, partial nestlings elimination can at least ensure the nest success; moreover, sacrificing these nestlings might increase the parents' future reproductive prospects. By contrast, Grey-backed Shrikes in our study area raise only one brood a year. Hence, any starving nestling will reduce parental fitness, whereas sacrificing these nestlings seems unlikely to increase their future reproductive prospects because they produce fixed-sized clutches. Therefore, it is most beneficial for Grey-backed Shrikes to raise the entire brood in the current reproduction. In altricial birds, once hatching asynchrony occurs and size hierarchy is established within the brood, later offspring will be disadvantaged when competing for food with their older brood-mates (Du et al. 2012; Fan et al. 2017). However, we found the last Grey-backed Shrikes offspring to have the highest growth rate within the brood (Fig. 2). Although we obtained no direct behavioral evidence for it, we believe it to be a consequence of parental brood survival strategy. If the last nestlings had not obtained a larger food supply than the other nestlings, they could not keep up with the older nestlings' growth.
The wide range of Grey-backed Shrike dietary items ensures that parents could support their entire brood. The dietary investigation revealed that parents delivered eleven types of food to their nestlings (Fig. 4), which cover the most common insects found in our study area (Li et al. 2020a) and some plant food types that are also used by other sympatric birds (Fan et al. 2017; Li et al. 2020a). The Grey-backed Shrike's wide range of dietary items underlies their high provisioning rate and amount of food delivered per feeding bout. As a result, Grey-backed Shrikes can adopt the brood survival strategy in provisioning their offspring.
Life-history style underlying the parenting strategy of altricial birds
Differences in life-history style between sympatric birds, such as the Brown-cheeked Laughing Thrush and Grey-backed Shrike in our study area, could explain the differences in their parenting strategies. The Brown-cheeked Laughing Thrushes have a longer breeding season from early April to later September (Li et al. 2020b). They can, therefore, breed twice a year and adopt different parenting strategies in the two breeding attempts. For example, they deliver food evenly to the nestlings early in the breeding season (brood survival strategy), while bias food towards larger offspring later in the breeding season (brood reduction strategy; Li et al. 2020b). In contrast, Grey-backed Shrikes have a shorter breeding season from the end of June to early September. Hence, they can breed only once a year, so that a brood survival strategy could maximize their reproductive success. Under these conditions, consistent parenting strategies should be maintained between the egg and nestling phases.
Other behavioral responses to the local environmental conditions driven by the one brood a year life-history style also underlie the Grey-backed Shrike brood survival strategy. First, Grey-backed Shrike parents initiate their reproduction in late June, when most berries are ripe (Fan et al. 2017). A large proportion of the sympatric shrub-nesting birds' diet, including the Brown-cheeked Laughing Thrush and White-collared Blackbird, is composed plant feed (Fan et al. 2017; Li et al. 2020b), indicating that the Grey-backed Shrike dietary composition differ from that of multi-brooded species in the area (Fig. 4). The Grey-backed Shrikes can broaden the range of items in their diet and thus reduce the competition with sympatric species. Moreover, the Brown-cheeked Laughing Thrushes and White-collared Blackbirds exhibit sexual division in provisioning the brood (Fan et al. 2017; Li et al. 2020b). The females often contribute less to provisioning the first brood, whereas the males often contribute less to provisioning the second brood, because they made a greater contribution to provisioning the first one. In contrast, Grey-backed Shrikes do not exhibit such sexual differences because both parents contribute equally to the total food supplied. Sexual division in brood provisioning would make it impossible for the Grey-backed Shrike parents to raise the entire brood by adopting a brood survival strategy.
Conclusions
By investigating the egg-laying and nestling growth pattern, we determined that the Grey-backed Shrike parents adopt the brood survival strategy during their parenting process. The one brood a year life-history style in this species, the delayed initiation of reproduction, and the absence of sexual division in brood provisioning might underlie the brood survival strategy adopted by the Grey-backed Shrike parents.
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1186/s40657-021-00244-x.
Acknowledgements
We are grateful to Lili Xian, Juanjuan Luo, Guoliang Chen and Xinwei Da for their help in the field work.
Authors’ contributions
BD and LF designed the study. LG, ZZ, XZ, HZ, WZ and JL collected the life-history data in fieldwork. BD and LG analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Availability of data and materials
The data used in the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The ethical permits for the nest translocation experiment were issued by the Tibetan Forestry Department (2016ZR-NY-05). The procedures of animal measurement are under the Wildlife Conservation Law of P. R. China (20170101).
Consent for publication
Not applicable.
Competing interests
All authors declare no conflicts of interest to any other organization bodies.
-
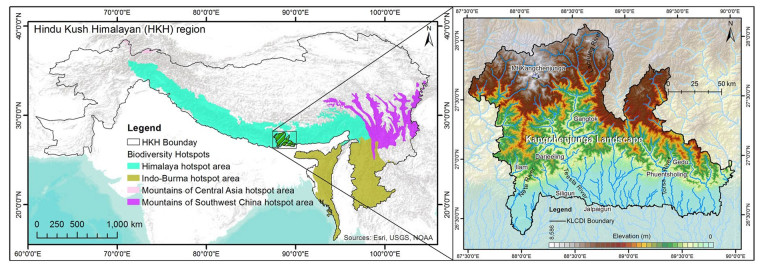
Table 1 The Kangchenjunga Landscape area by country and percentage of total area
Country Area (km2)* Percentage of total KL area* (%) Total number of species Number of threatened species Bhutan 5834 23 210 4 India 14, 062 56 585 39 Nepal 5190 21 342 15 Total 25, 086 100 *Source ICIMOD et al. (2017), other data from this study Table 2 Threat status of birds of the Kangchenjunga Landscape
Threat status Number of species Percentage (%) Critically endangered 5 0.81 Endangered 3 0.49 Vulnerable 19 3.07 Near threatened 14 2.27 Least concern 577 93.37 Table 3 Important Bird and Biodiversity Areas in the Kangchenjunga Landscape
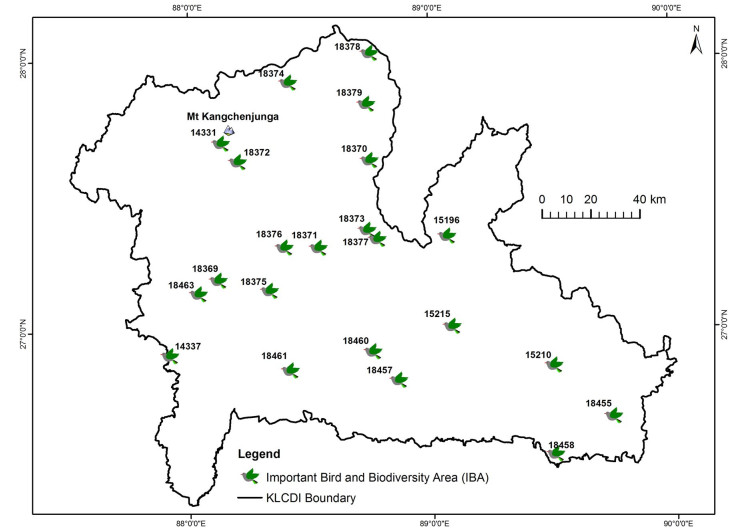
IBA* Country Protected area Significant bird species Jigme Khesar Strict Nature Reserve (BT002) (formerly known as Toorsa Strict Nature Reserve) Bhutan √ Chestnut-breasted Partridge Wood Snipe Rufous-necked Hornbill Samtse (BT003) Bhutan Rufous-necked Hornbill Chele La (BT004) Bhutan Wood Snipe Paro wetlands (BT005) Bhutan Wood Snipe Kamji (BT007) Bhutan Rufous-necked Hornbill Lava-Neora Valley National Park (IN322) India √ Eastern-imperial Eagle Pale-capped Pigeon Rufous-necked Hornbill Black-breasted Parrotbill Beautiful Nuthatch Mahananda Wildlife Sanctuary (IN323) India √ White rumped Vulture Slender-billed Vulture Bengal Florican Swamp Francolin Lesser Adjutant Rufous-necked Hornbill Black-breasted Parrotbill Singhalila National Park (IN325) India √ Chestnut-breasted Partridge Greater-spotted Eagle Wood Snipe Beautiful Nuthatch Barsey Rhododendron Sanctuary (IN327) India √ Pallas's Fish-eagle Black-breasted Parrotbill Dombang Valley-Lachung-Lema-Tsungthang (IN328) India Wood Snipe Beautiful Nuthatch FambongLho Wildlife Sanctuary-Himalayan Zoological Park-Ratey Chu Reserve Forest (IN329) India √ White rumped Vulture Slender-billed Vulture Chestnut-breasted Partridge Rufous-necked Hornbill Beautiful Nuthatch Khangchendzonga National Park and Biosphere Reserve (IN330) India √ Baer's pochard Pallas's Fish-eagle Black-breasted Parrotbill Kyongnosla Alpine Sanctuary-Tsomgo-Tamze-Chola Complex (IN331) India √ Greater-spotted Eagle Pallas's Fish-eagle Wood Snipe Lhonak Valley (IN332) India Wood Snipe Black-necked Crane Lowland forests of South Sikkim (IN333) India White rumped vulture Slender-billed Vulture Chestnut-breasted Partridge Rufous-necked Hornbill Grey-crowned Prinia Slender-billed Babbler Black-breasted Parrotbill Beautiful Nuthatch Maenam Wildlife Sanctuary-Tendong Reserve Forest (IN334) India √ Chestnut-breasted Partridge Blyth's Tragopan Greater-spotted Eagle Rufous-necked Hornbill Beautiful Nuthatch Pangolakha Wildlife Sanctuary-Zuluk-Bedang Tso-Natula Complex (IN335) India √ Chestnut-breasted Partridge Greater-spotted Eagle Pallas's Fish-eagle Wood Snipe Rufous-necked Hornbill Grey-crowned Prinia Slender-billed Babbler Black-breasted Parrotbill Tso Lhamo Plateau-Lashar-Sebu La-Yumesandong Complex (IN336) India Greater-spotted Eagle Wood Snipe Black-necked Crane Yumthang-Shingba Rhododendron Wildlife Sanctuary (IN337) India √ Wood Snipe Kangchenjunga Conservation Area (NP010) Nepal √ Wood Snipe Spiny Babbler Mai Valley Forests (NP015) Nepal White Rumped Vulture Slender-billed Vulture Red-headed Vulture Wood Snipe Greater-spotted Eagle Lesser Adjutant Spiny Babbler Tamur Valley and Watershed (NP026) Nepal Spiny Babbler *Numbers indicate IBA code numbers. The Latin names of all these species are in Additional file 2 Table 4 A summary of national policies, laws and international conventions of the countries that share the Kangchenjunga Landscape (in chronological order)
Bhutan India Nepal Some national policies National Forest Policy 1974 Forest Policy 1952 New Forest Policy 1978 Master plan for forest development 1990 National conservation Strategy and Policy Statement, India 1992 National Conservation Strategy 1988 which is later revisited as Nature Conservation National Strategic Framework for Sustainable development (2015-2030) Biodiversity Action plan 1994 National Forest Policy 1998 Environment Policy and action Plan 1993 National Environment Strategy ("The Middle Path") 1998 National Wildlife Action Plan (2002-2016) Tenth Plan (2002-2007) Biodiversity Action Plan 2002 National Action Plan on Climate Change 2008 Nepal Biodiversity Strategy 2002 Revision of Forest Policy draft 2010 National Biodiversity Strategy and Action Plan 2009 Sustainable Development Agenda for Nepal 2003 Nepal Biodiversity Strategy Implementation Plan 2006 National Bio-safety framework 2006 Three-year Interim Plan (2007-2010) National Agriculture Policy 2004 Rangeland Policy 2012 National Wetland Policy 2012 Vulture Conservation Action Plan (2009-2013) National Biodiversity Strategy and Action Plan (2014-2020) Some national laws Bhutan Forest Act 1969 Indian Forest Act 1927 and its successive amendments 1980 Nepal Legal Code "the muliki ain" 1854 Forest and Nature Conservation Act 1995 Wildlife (Protection) Act 1972 (last amended in 2013) Nepal Forest Nationalization Act 1957 Environment Assessment Act 2002 Environment Protection Act 1986 Nepal Forest Act 1962 its amendments 1968 Forest and Nature Conservation Rules Volumes I & II 2002 Panchayati Raj (Extension to Scheduled Areas) Act 1996 Forest Act 1993 National Biodiversity Act 2003 Biological Diversity Act 2002 National Parks and Wildlife Conservation Act, Nepal 1972 and amendment 2002 Land Act of Bhutan 2007 National Environment Protection Act 2007 International conventions Ramsar Convention 1971 Ramsar Convention 1971 Ramsar convention 1971 United Nations Convention on International Trade in Endangered Species of Wild Flora and Fauna (CITES) 1975 UNESCO's Man and Biosphere Programme 1971 CITES Convention on Biological Diversity (CBD) 1992 CITES 1975 Bonn convention 1983 United Nations Framework Convention on Climate Change (UNFCCC) 1992 Convention on the Conservation of Migratory Species of Wild Animals (Bonn Convention) 1983 CBD 1992 United Nations Convention to Combat Desertification (UNCCD) 1994 CBD 1992 UNFCCC 1992 UNFCCC 1992 UNCCD 1994 UNCCD 1994 Kyoto Protocol to the UNFCCC IBAs 23 (8 within protected area network) 465 (279 with in protected areas network and 16 are listed as Ramsar sites) 27 (13 within protected areas network) -
-
期刊类型引用(7)
1. Pstrokoński, P., Roszkowiak, Ł., Korzyńska, A. et al. Can explainable AI classify shrike (Laniidae) eggs by uncovering species-wide pigmentation patterns?. Plos One, 2025, 20(5 May): e0321532.  必应学术
必应学术
2. Jia, B.-Y., Zhu, Z.-Q., Zhu, W.-J. et al. Offspring number and composition influence parental care strategy and offspring survival in the azure-winged magpie. Animal Behaviour, 2025.  必应学术
必应学术
3. Zhang, X., Zhang, Z., Lu, W. et al. Extra-pair paternity enhances the reproductive fitness of urban Chinese blackbird. Journal of Avian Biology, 2025, 2025(1): e03129.  必应学术
必应学术
4. Dolenec, Z.. REPRODUCTIVE STRATEGY OF THE SONG THRUSH Turdus philomelos IN RELATION TO EGG DIMENSION | [Strategija razmnožavanja drozda cikelja Turdus philomelos u odnosu na dimenzije jaja]. Larus Godisnjak Zavoda Za Ornitologiju Hrvatske Akademije Znanosti I Umjetnosti, 2024, 59(1): 47-51.  必应学术
必应学术
5. Zhu, Z.-Q., Zi, S.-M., Gao, L.-F. et al. A diagnosis model of parental care: How parents optimize their provisioning strategy in brood reduction?. Current Zoology, 2023, 69(4): 385-392.  必应学术
必应学术
6. Liu, F., Gao, L., Wang, Q. et al. Giant babax (Babax waddelli) helpers cheat at provisioning nestlings in poor conditions. Behavioral Ecology and Sociobiology, 2023, 77(1): 16.  必应学术
必应学术
7. Ritchison, G., Lewis, L., Heist, C.A. Provisioning behavior of male and female Loggerhead Shrikes. Avian Biology Research, 2022, 15(2): 93-99.  必应学术
必应学术
其他类型引用(0)


 下载:
下载: